pKa in Reverse Phase HPLC governs peak elution. It plays a unique and critical role in the separation of analytes as it directly influences selectivity
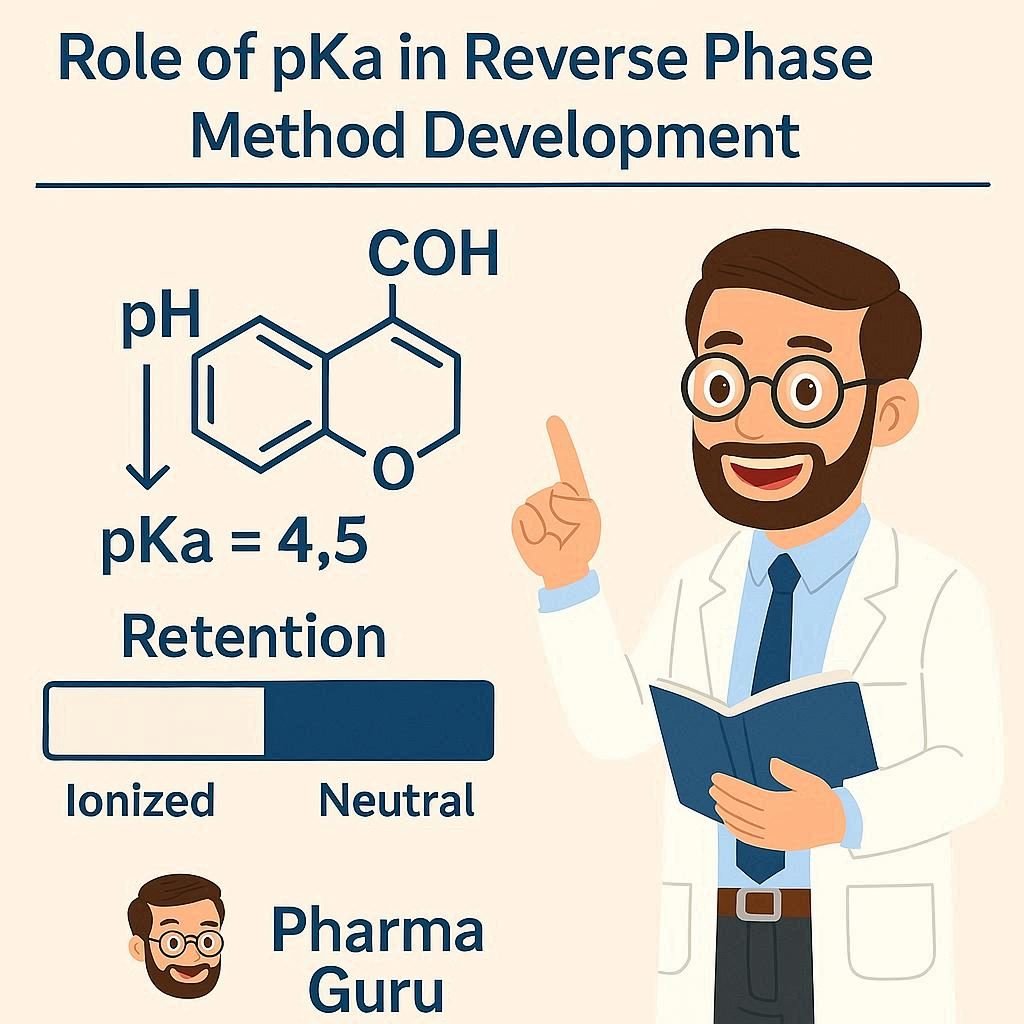
pKa in Reverse Phase HPLC governs peak elution. It plays a unique and critical role in the separation of analytes as it directly influences selectivity. It is one of the key factors in method development, determining the ionisation state of analytes and thus affecting their retention and resolution. But what exactly is pKa, and why is it so important in RP-HPLC?
pKa is the negative base-10 logarithm of the acid dissociation constant (Ka) of a molecule. In simpler terms, it tells us the pH at which a molecule exists 50% in its ionised form and 50% in its unionised form.
Knowing the pKa allows chemists to predict the ionisation state of a molecule at a given pH.
| Buffer | pKa1 | pKa2 | pH Range | Usage |
|---|---|---|---|---|
| Acetic acid / Acetate | 4.76 | 3.8 – 5.6 | Common for reverse-phase HPLC, weak acidic buffer | |
| Phosphate Buffer | 7.2 (H₃PO₄) | 7.6 (H₂PO₄⁻ / HPO₄²⁻) | 6.0 – 8.0 | Widely used for biological samples, neutral pH |
| Citric Acid / Citrate | 3.13 | 5.60 | 2.2 – 7.0 | Used for low pH, ion exchange columns |
| Tris (Tris(hydroxymethyl)aminomethane) | 8.1 | 7.0 – 9.0 | Buffers for biological or biochemical samples, slightly basic | |
| Ammonium Acetate | 9.25 | 7.0 – 9.5 | Used in mass spectrometry applications, good for basic pH | |
| Formic Acid / Formate | 3.75 | 2.0 – 4.0 | Often used in reverse-phase HPLC for better ionization in LC-MS | |
| Borate Buffer | 9.14 | 8.0 – 10.0 | Used in anion-exchange chromatography, often in alkaline pH | |
| HEPES (N-2-Hydroxyethylpiperazine-N’-2-ethanesulfonic acid) | 7.5 | 8.1 | 6.8 – 8.2 | Common for biological or biochemical studies |
| Sodium Bicarbonate (NaHCO₃) | 10.3 | 8.5 – 11.0 | Used in alkaline pH settings, common for ion-exchange chromatography |
1. Ionisation State Affects Retention
In RP-HPLC, the stationary phase is nonpolar (e.g., C18), and the mobile phase is polar (usually water mixed with an organic solvent like acetonitrile or methanol). The retention of compounds in this system is primarily driven by hydrophobic interactions.
By adjusting the pH of the mobile phase relative to the compound’s pKa, you can control how much of the compound is ionised, thereby controlling its retention time.
2. Choosing the Right pH for Selectivity
In method development, achieving good resolution between peaks is key. Manipulating pH can help separate closely eluting compounds by changing their relative retention based on differing pKa values.
For example:
3. Ensuring Peak Shape and Efficiency
Operating the mobile phase at a pH close to the analyte’s pKa can lead to peak tailing or broadening, due to mixed populations of ionised and unionised species. To avoid this:
In RP-HPLC method development, understanding the pKa of your analyte is not optional—it’s essential. It directly influences retention time, resolution, and peak shape. By strategically selecting the mobile phase pH based on pKa, you gain a powerful lever to control and optimise your separation.
You May Like
pKa is important in HPLC because it determines the ionisation state of analytes at a given pH, which directly affects their retention time, separation, and peak shape. By adjusting the mobile phase pH relative to the analyte’s pKa, you can optimise selectivity and improve resolution in the chromatographic method.
Further Reading
Quick Links