Process validation and product validation are distinct yet complementary activities essential to ensuring quality in pharmaceutical manufacturing. Process validation confirms that the manufacturing process consistently yields products meeting predefined specifications, while product validation verifies that the final product meets its intended use and quality requirements. In pharmaceutical development, both the drug and its manufacturing process […]
Process validation and product validation are distinct yet complementary activities essential to ensuring quality in pharmaceutical manufacturing. Process validation confirms that the manufacturing process consistently yields products meeting predefined specifications, while product validation verifies that the final product meets its intended use and quality requirements.
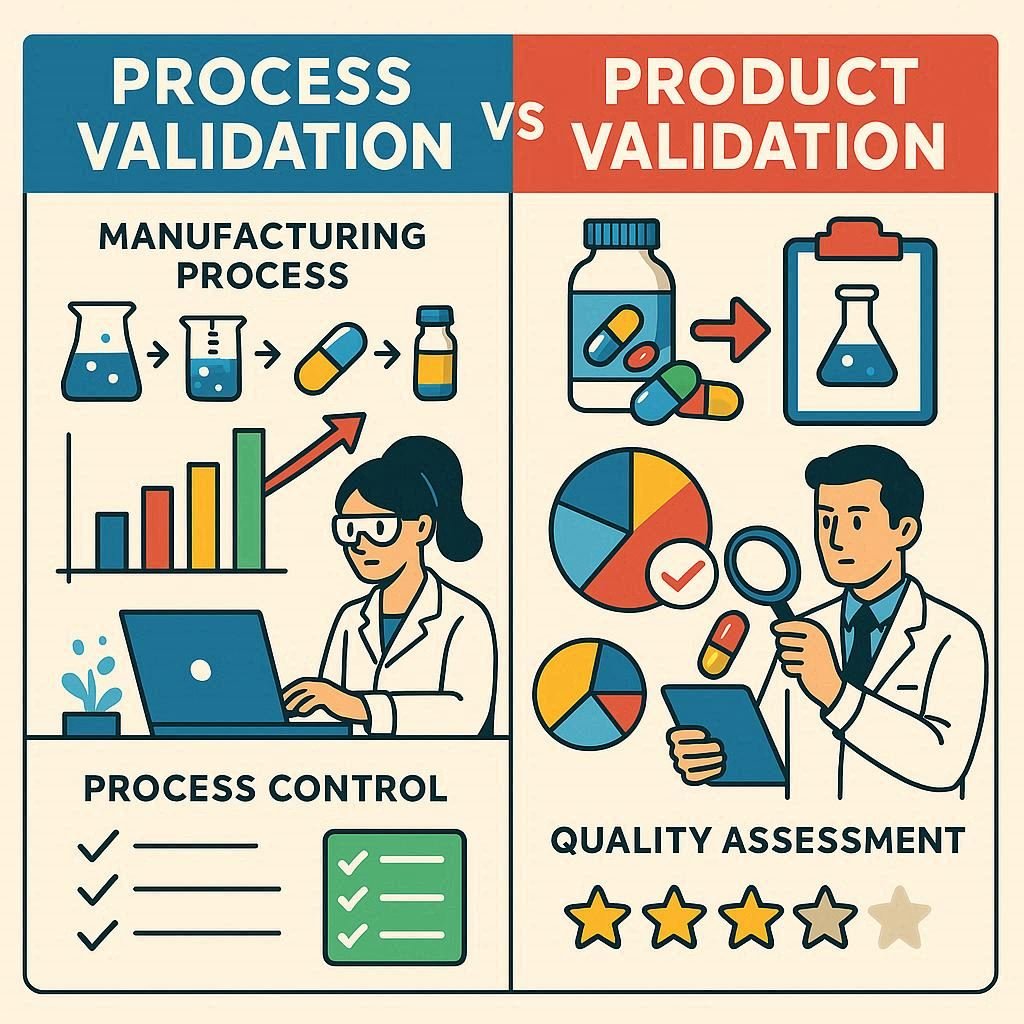
In pharmaceutical development, both the drug and its manufacturing process must meet strict quality and safety standards. Process Validation and Product Validation are key components of quality assurance, each serving a distinct role. Understanding their differences is vital for regulatory compliance, product quality, and patient safety.
Major Takeaway: FAQs
The four main types of validation in pharmaceuticals are:
1. Prospective Validation – Conducted before a new process is implemented.
2. Concurrent Validation – Performed during the actual production of products intended for sale.
3. Retrospective Validation – Based on historical data from past production batches.
4. Revalidation – Repeated validation to ensure continued process control, often after changes or over time.
In APQP (Advanced Product Quality Planning):
1. Process Validation ensures that the manufacturing process can consistently produce products that meet specifications.
2. Product Validation confirms that the final product meets all customer requirements and functions as intended under real-world conditions.
Phase 4 of APQP – Product and Process Validation involves:
1. Verifying that the product meets all design and customer requirements.
2. Validating that the manufacturing process consistently produces conforming parts at planned production rates.
This phase includes production trial runs, capability studies, and submission of the PPAP (Production Part Approval Process).
Process Validation is the documented evidence that a manufacturing process will consistently produce a product that meets predetermined specifications and quality attributes. It focuses on the how—how the drug is made, and whether that method is reliable and reproducible.
Example: Validating the sterilization process for injectable drugs to ensure that every batch is free from microbial contamination.
Product Validation, sometimes called Product Qualification, focuses on the what—verifying that the final pharmaceutical product meets the required quality standards, specifications, and intended use.
Example: Testing tablets for correct dosage, dissolution rate, and absence of harmful impurities.
You May Like:
| Aspect | Process Validation | Product Validation |
|---|---|---|
| Focus | Manufacturing process | Final pharmaceutical product |
| Purpose | To ensure process consistency and control | To ensure product quality, safety, and efficacy |
| Timing | Throughout the lifecycle of production | Typically after manufacturing, during QC and release |
| Scope | Equipment, systems, procedures, and process parameters | Typically, after manufacturing, during QC and release |
| Outcome | Robust, reproducible manufacturing process | Safe and effective drug that meets all regulatory requirements |
A validated process without product validation can still produce poor-quality drugs if something goes wrong post-manufacture. Conversely, a product might pass quality checks sporadically, but without a validated process, there’s no guarantee it will do so consistently.
Regulatory agencies like the FDA and EMA expect pharmaceutical companies to have both types of validation in place. Together, they form the backbone of Good Manufacturing Practices (GMP) and help protect patient health.
In summary, Process Validation ensures that pharmaceutical products are made correctly, while Product Validation ensures that what’s made works correctly. Both are essential, interdependent pillars of quality assurance in the pharmaceutical industry.
Whether you’re a manufacturing professional, quality expert, or regulatory specialist, understanding and implementing both types of validation is key to maintaining compliance and delivering safe, effective medications to the market.
Related:
Further Reading:
Quick Links