While the ICH and ANVISA method validation guidelines share the same fundamental goal of ensuring reliability and accuracy, they differ in their level of detail and regulatory expectations. ANVISA generally adopts a more stringent approach, requiring the use of independent stock solutions for linearity assessments, more rigorous statistical analyses (including ANOVA and homoscedasticity testing), and […]
While the ICH and ANVISA method validation guidelines share the same fundamental goal of ensuring reliability and accuracy, they differ in their level of detail and regulatory expectations. ANVISA generally adopts a more stringent approach, requiring the use of independent stock solutions for linearity assessments, more rigorous statistical analyses (including ANOVA and homoscedasticity testing), and specific stress conditions in forced degradation studies—such as metal ion oxidation. Additionally, ANVISA provides more comprehensive requirements for robustness testing, stability studies, matrix effect evaluation, and reanalysis of incurred samples, whereas the ICH guidelines take a broader, more principle-based approach
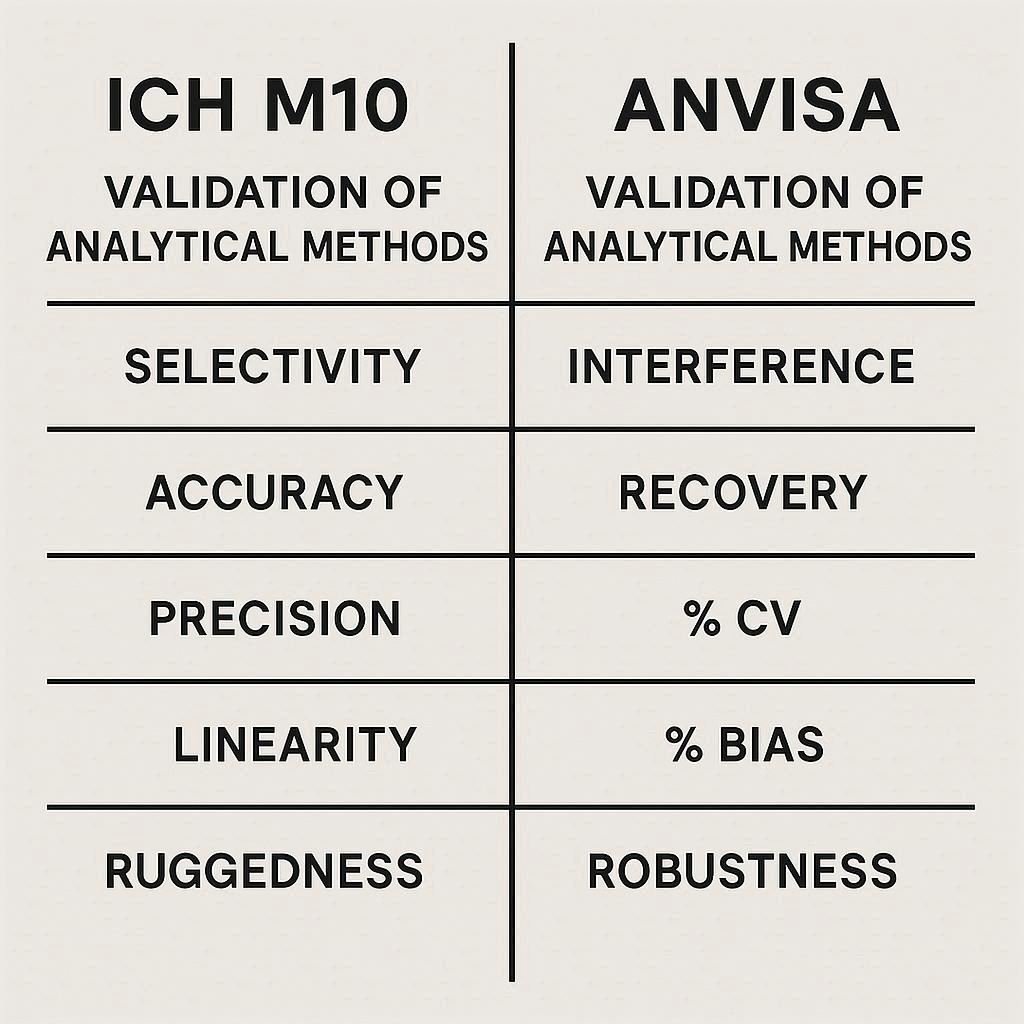
Method validation ensures that an analytical procedure is reliable, accurate, reproducible, and suitable for its intended purpose.
Yes, ICH guidelines are internationally harmonised and adopted by regulatory authorities in regions including the US, EU, Japan, and many other countries.
Yes, ANVISA mandates specific forced degradation studies, including stress conditions like oxidation, heat, light, and metal ions, to demonstrate method specificity and stability-indicating capability.
ANVISA emphasises rigorous statistical analysis, including ANOVA, homoscedasticity testing, regression evaluation, and residual analysis for method validation parameters like linearity and precision.
Yes, but additional requirements may need to be met, such as stricter linearity evaluation, independent stock solutions, robustness studies, and detailed documentation per ANVISA standards.
ANVISA guidelines are generally more detailed and stringent, with specific requirements for linearity, statistical analysis, forced degradation, robustness, and stability studies, while ICH guidelines are broader, principle-based, and internationally harmonized
ANVISA stands for Agência Nacional de Vigilância Sanitária, Brazil’s National Health Surveillance Agency.
Brazil.
ICH recommends using at least three concentration levels with triplicate measurements, whereas ANVISA requires five concentration levels, including the lower limit of quantification (LLOQ), along with more stringent criteria for intermediate precision.
You May Like
Linearity is one of the core analytical method validation parameters, and while both ICH Q2(R2) and ANVISA RDC 166/2017 cover it, they differ in procedural expectations, acceptance criteria, and statistical treatment.
| Aspect | ICH Q2(R2) | ANVISA RDC 166/2017 | Key Differences / Remarks |
|---|---|---|---|
| Definition of Linearity | Linearity is the ability of the method to obtain test results that are directly proportional to analyte concentration within a given range. | Same definition — ability of the method to obtain results directly proportional to analyte concentration in a specific range. | Conceptually identical. |
| Concentration Range | At least 5 concentration levels covering 80–120% of the expected working range. | Requires at least 5 concentration levels, typically 50–150% of target concentration (wider than ICH). | ANVISA suggests broader coverage to verify method robustness. |
| Replicates | Replicates not strictly required — can use single measurements per level if justified. | Recommends at least 3 replicates per level for improved statistical confidence. | ANVISA explicitly requires replicates; ICH allows flexibility. |
| Plot Requirements | Plot of response vs. concentration recommended (visual inspection). | Same definition — the ability of the method to obtain results directly proportional to analyte concentration in a specific range. | ANVISA specifies detailed documentation requirements. |
| Documentation | Report slope, intercept, correlation coefficient, residuals, and linearity range. | Must include raw data, calculations, plots, statistical analysis (ANOVA), and acceptance decision. | ANVISA documentation requirements are more comprehensive. |
When it comes to forced degradation studies, ICH guidelines provide general principles but do not prescribe specific degradation conditions, leaving the design of stress studies to scientific justification. In contrast, ANVISA takes a more prescriptive approach, requiring specific stress conditions to ensure the method’s ability to indicate stability. Notably, ANVISA mandates metal ion oxidation using copper (II) and iron (III) ions as part of the oxidative stress evaluation. Additionally, ANVISA recommends achieving a minimum of 10% degradation under each stress condition to confirm method sensitivity—though a lower degradation level can be justified for highly stable molecules. This difference reflects ANVISA’s emphasis on demonstrating method robustness and comprehensive degradation profiling compared to ICH’s more flexible, science-based framework.
Method validation is a critical part of ensuring the reliability, accuracy, and consistency of analytical data in pharmaceutical development and quality control. While both ICH and ANVISA provide structured frameworks for validation, there are subtle differences in focus, terminology, and regional expectations.
The table below summarises the key similarities and differences between the two.
| Aspect | ICH Guidelines | ANVISA Guidelines | Key Takeaway |
|---|---|---|---|
| Reference Document | ICH Q2(R2) – Validation of Analytical Procedures (latest revision, 2023) | RDC No. 166/2017 – Validation of Analytical Methods and Bioanalytical Methods | Recommended as part of the routine method control. |
| Scope | Applies to drug substance and product testing methods for registration in ICH regions. | Applies to all pharmaceutical products registered in Brazil, including imported ones. | Both cover analytical validation, but ANVISA includes additional administrative aspects. |
| Types of Methods Covered | Identification, assay, impurity testing, and quantitative limit tests. | Identification, quantification, purity, dissolution, and bioanalytical methods. | ANVISA includes explicit coverage of bioanalytical and dissolution tests. |
| Validation Parameters | Accuracy, precision, specificity, detection limit, quantitation limit, linearity, range, robustness. | Same as ICH, plus selectivity and system suitability are more strongly emphasized. | Core parameters align closely, but ANVISA expands certain requirements. |
| Acceptance Criteria | Provides general recommendations and leaves numerical limits to the applicant. | Defines specific numerical acceptance criteria for precision, accuracy, and linearity. | ANVISA is more prescriptive; ICH is principle-based. |
| System Suitability | Recommended as part of routine method control. | Mandatory to demonstrate before method validation. | ANVISA treats it as a formal prerequisite. |
| Requires a detailed documentation format for submission to ANVISA. | Revalidation required when major method or process changes occur. | Defines full, partial, and cross-validation explicitly. | ANVISA gives more structured guidance for partial validations. |
| Documentation Requirements | Emphasis on scientific justification and traceability. | ANVISA demands a more formal documentation structure. | ANVISA demands more formal documentation structure. |
| Statistical Treatment | Encourages statistical analysis but allows flexibility. | Specifies certain statistical tests (e.g., ANOVA for precision). | ANVISA provides more explicit statistical requirements. |
| Robustness and Ruggedness | Robustness must be evaluated; ruggedness considered optional. | Both robustness and ruggedness must be demonstrated. | ANVISA explicitly differentiates and requires both. |
| Bioanalytical Validation | Covered separately in other ICH guidance (e.g., M10). | Included within RDC 166/2017 with clear criteria for matrix effect, stability, etc. | ANVISA integrates bioanalytical validation into its main regulation. |
| Regulatory Submission | Used by EMA, FDA, PMDA, and other ICH member countries. | Required for all submissions to ANVISA in Brazil. | ICH has international scope; ANVISA applies nationally. |
| Approach | Science-based, risk-oriented, flexible for innovation. | Compliance-driven, documentation-intensive, and aligned with Brazilian GMP. | ICH promotes harmonization and flexibility; ANVISA focuses on compliance and transparency. |
Both ICH Q2(R2) and ANVISA RDC 166/2017 share the same scientific foundation for analytical method validation. However:
In practice, methods validated under ICH Q2(R2) can often be accepted by ANVISA with minor adjustments — especially in reporting format, statistical rigour, and system suitability testing.
When planning analytical validation for global submissions, it’s wise to design your protocol to satisfy both ICH and ANVISA simultaneously. This ensures smoother approval across international and Brazilian markets while maintaining scientific integrity.
Further Reading
Quick Links