
Learn about GCMS in drug development; its principles, applications in industries, real-world case studies, and answers to frequently asked questions
GCMS (Gas Chromatography-Mass Spectrometry is the combination of a Gas chromatograph and Mass Spectrophotometer
It is a highly sensitive, accurate and reliable analytical technique, widely employed in drug development for both qualitative and quantitative analysis. Its exceptional sensitivity makes it particularly valuable for detecting and quantifying impurities, especially at trace levels, including nitrosamines and other genotoxic compounds.
In this article, I will provide skill-based insights into the principles, applications, advantages, limitations, case studies, and frequently asked questions related to GC-MS. By the end of this article, you will be well-equipped to develop GC-MS methods and predict the structure of unknown compounds during various stages of drug development.
GC-MS is a unique combination of two powerful techniques. The first technique is the GC instrument with a capillary column, and the second technique is the mass detector. The function of GC is to separate different components from the sample mixture and send them one by one to the MS detector. The function of the MS detector is to break down each component into its different fragments (minor and major). These different fragments give structural information about the component. The major fragment is also used for quantification of the component.
GCMS is the combination of a Gas chromatograph and Mass Spectrophotometer (Figure-1)
GCMS = GC + MS
Figure-1

Related:
GC-MS is the integration of GC with a capillary column and an MS detector
The Electron ionisation (EI) and chemical ionisation (CI) are used in most of the GC-MS instruments:
It is also called hard ionisation, and it is widely used for structure elucidation. The molecule which enters into the EI-ion source gets electron bombarded of 70 EV. Due to this bombardment molecule breaks down into different fragments. The pattern of this fragment is highly specific and acts as a fingerprint, which is used for the structure characterisation of the unknown compound. A stable (M+) or an unstable (M*) molecular ion may form due to this bombardment
M +e– → M+
M + e– → M* →M1+ or M2+ or M3+
It is also called soft ionisation, and it is used for mass determination. In this process, methane gas is passed at high pressure in the CI source. Since methane has a very low proton affinity* and hence it can transfer a proton to any molecule, and that is the reason it is used in the CI source for ionisation of the molecule. The CI process may go by the following mechanism:
Proton transfer
M +CH5+ →[M-H]+ + CH4 and so on
Note: Proton affinity for methane is 549/kJ/mol
| EI ionisation | CI ionisation |
| It is hard ionisation | It is soft ionisation |
| It is used only for the mass determination of a molecule | It is used only for the mass determination of a molecule |
| In EI ionisation molecule gets electron bombarded of 70 EV. | In CI ionisation, methane gas is passed at high pressure |
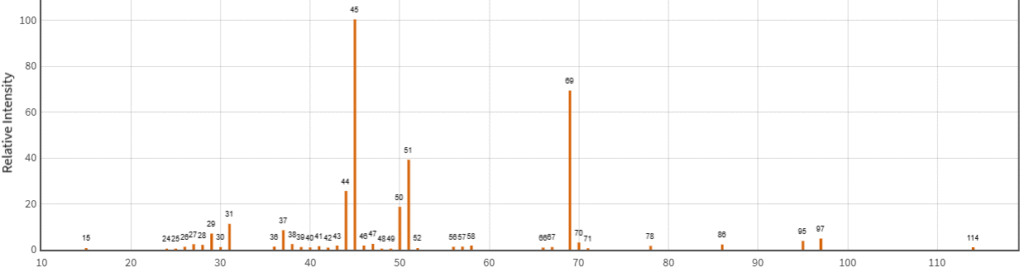
In GC-MS, the x-axis represents the m/z ratio, and the y-axis represents the relative intensity of the fragment (Figure-2)
Figure-2:
SIM mode or selected ion monitoring mode allows the mass spectrometer to detect specific compounds with high intensity. In this mode single fragment is monitored. It is mainly used for quantification purposes.
MRM, or multiple reaction monitoring mode is used to collect data on different fragments of the molecule. It is used for structural characterisation purposes
The following are the various applications of GCMS in the Pharmaceutical industry:
Apart from pharmaceutical industries, GCMS is the the following industries for various tests:
The following are the advantages of the GC-MS:
The following are the disadvantages of the GC-MS:
Use the following steps to identify the structure:
An unknown compound that gives major fragments of 29D, 43D, 45D, 51D and 60D in EI mode. In CI mode, it gives the mass of 60D.
The EI spectrum predicts the closest structure of Acetic acid.
Molecular ion (M⁺)
The molecular ion for acetic acid is typically observed at m/z 60: CH3COOH+ (m/z 60)
Loss of the hydroxyl group (COOH → CO)
One common fragmentation pathway is the loss of the -OH group from the carboxyl group. This produces an ion at: CH3CO+(m/z 43)
This fragment is stable and often one of the most prominent peaks in the EI mass spectrum. I
Acetic acid can also undergo decarboxylation, losing a molecule of carbon dioxide (CO₂). This process is common for carboxylic acids. The resulting fragment ion is: CH3+(m/z 15)
This is a methyl ion, a very stable species. Decarboxylation is a strong fragmentation pathway for carboxylic acids, and this peak is often observed.
Cleavage of the C–C bond (Formation of C₂H₅⁺)
Another fragmentation pathway involves the cleavage of the C–C bond in acetic acid. This produces an ethyl ion C2H5+(m/z 29)
The control, quantification, characterisation, and identification of highly carcinogenic compounds would be extremely challenging – if not impossible – without the use of GC-MS. This is why GC-MS plays a crucial role in pharmaceutical research and development. Hopefully, this post has helped clarify the importance of GC-MS and deepened your understanding of its applications
You may also want to check out other articles on my blog, such as:
GC-MS is used for mass determination, identification, quantification and characterisation of unknown or known compounds
GC-MS is the combination of GC with a capillary column and mass. GC separate the different components of the sample mixture and mass identifies each component.
GC-MS is the combination of GC with a capillary column and mass spectrometry. GC separate the different components of the sample mixture and mass identifies each component.
Due to its sensitivity, reliability, precision and accuracy, GC-MS is best for drug analysis
GCMS is highly sensitive, reliable, precise and accurate technique
EI gives structural information about the molecule, whereas CI gives mass of the molecule
Mass spectrometry is an analytical technique used to measure the mass-to-charge ratio (m/z) of ions in a sample. These measurements can be used to determine the exact molecular weights of the sample’s components
Gas chromatography–mass spectrometry (GC–MS) is a powerful analytical technique that combines the separation capabilities of gas chromatography with the identification power of mass spectrometry to detect, identify, and characterise compounds within a sample
Quick Links