Learn what enantiomeric excess is, how it’s calculated, and its real-world applications with case studies.
Enantiomeric excess (ee) determines the purity and performance of chiral compounds. In this Blog, I will discuss what enantiomeric excess is, how it’s calculated, and its real-world applications with case studies.
Enantiomeric excess (ee) is a quantitative measure of the purity of a chiral substance in terms of its enantiomeric composition. It indicates how much one enantiomer is present in excess over the other in a mixture of two enantiomers. Expressed as a percentage, enantiomeric excess reflects the degree to which a sample deviates from a racemic (50:50) mixture and helps determine the dominance of one enantiomer in the sample.
A racemic mixture (50:50 of both enantiomers) has an ee of 0%, while a single completely pure enantiomer has an ee of 100%. A sample with 65% of one enantiomer and 35% of the other has an ee of 30% (65% − 35%).
Note: A chiral molecule can exist as two non-superimposable mirror images—known as enantiomers—typically labelled as (R) and (S).
The Enantiomeric Excess is calculated by the following formula:
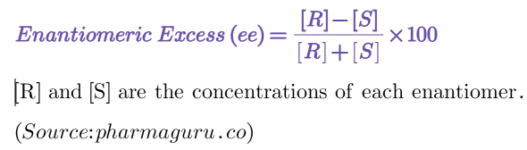
Where:
If you know the % of each enantiomer:
Enantiomeric Excess (ee)=∣%Major Enantiomer−%Minor Enantiomer∣
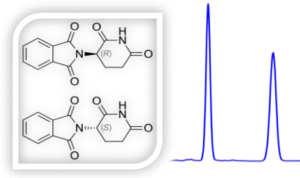
The above sample contains 80% of the R-enantiomer and 20% of the S-enantiomer.
Therefore, Enantiomeric Excess (ee) =80%−20%=60%
This means the sample is 60% enriched in the R-enantiomer.
Related:
Enantiomeric excess is more than a number—it’s a vital parameter in ensuring the safety, efficacy, and sustainability of chiral substances. Whether you’re developing a new drug or analysing a compound’s purity, understanding and calculating ee is foundational in modern chemistry.
Related:
n most contexts, enantiomeric excess and optical purity are used interchangeably. However, optical purity specifically refers to how much the observed optical rotation deviates from that of a pure enantiomer, while enantiomeric excess is based on the molar or percentage difference between enantiomers. Optical purity assumes the specific rotation of the pure enantiomer is known and constant.
It means the sample contains only one enantiomer and no trace of the other. In other words, it is optically pure.
Yes. A racemic mixture contains 50% of each enantiomer, so there’s no excess of either, resulting in 0% enantiomeric excess.
Answer: No. Enantiomeric excess is always expressed as a positive percentage. However, you can indicate which enantiomer is in excess (e.g., “60% ee in favor of the R-enantiomer”).
The following analytical techniques are used to measure ee:
Answer: Different enantiomers can have different biological effects. One may be therapeutic while the other is inactive or harmful. High ee ensures efficacy, safety, and regulatory compliance.
Answer: Yes, if the enantiomers interconvert under certain conditions (e.g., acidic or basic environments, heat), the ee can decrease over time. This is known as racemisation.
Answer: Diastereomeric excess (de) measures the difference between diastereomers, which are stereoisomers not related as mirror images. Enantiomeric excess (ee) is specific to mirror-image isomers.
Not always. Some drugs are sold as racemates if both enantiomers are safe or the body can convert one into the active form. However, the trend is moving toward enantiomerically pure drugs to optimize dosing and minimize side effects.
If you know the ee and the major enantiomer:
Example: If ee = 60% in favour of R:
Further Reading
Quick Links