Understand the role of capacity factor in HPLC, including how to calculate it, why it matters in method development, and what affects its value for optimal chromatographic results
Understanding the Capacity Factor in HPLC: A Key to Effective Method Development
The capacity factor in HPLC – also known as the retention factor (k’) – is a critical parameter that describes how well an analyte is retained on the column relative to an unretained or void volume peak. It provides insight into the interaction between the analyte and the stationary phase, serving as a foundational element in the separation process.
A proper understanding of the capacity factor is essential for successful chromatographic method development. It not only determines whether a compound is retained under given conditions but also significantly influences resolution and peak shape.
In this article, you will gain a clear understanding of:
By the end, you’ll be equipped with the knowledge to effectively evaluate and manipulate the capacity factor during method development for improved chromatographic performance.
The capacity factor (k’), also known as the retention factor, is a chromatographic parameter that indicates how long an analyte is retained on the column relative to an unretained (or void volume) peak. It reflects the extent of interaction between the analyte and the stationary phase under specific experimental conditions. A higher capacity factor suggests stronger retention. It is influenced by factors such as mobile phase composition, temperature, and the nature of the stationary phase. The capacity factor is typically denoted by the symbol k’ or K.
Related: How To Control Impurities In Pharmaceuticals: Get Mastery In …
The Following formula is widely used in the Pharmaceutical industry to calculate the Capacity factor:

Where:
The following factors affect the capacity factor in HPLC
Example-1: A moderately acidic compound having a capacity factor of 1.5 in the C18 column using a mixture of water and acetonitrile in a ratio of 40:60 and a flow rate of 1.0 ml/minute. to of the column is 0.5 minutes. How can more than 2 capacity factors can be achieved?
Discussion: A Higher capacity factor can be achieved by following adjustments in the chromatographic conditions:
Example-2: The following are the retention times of uracil and other acids on a C18 column (which is stable to 100% aqueous conditions), mobile phase 20 mM potassium phosphate buffer at pH 2.9 and with a flow rate of 0.7 ml/min.
| Peak | Name | RT (minutes) |
| 1 | Uracil | 1.5 |
| 2 | Acetic acid | 2.1 |
| 3 | Oxalic acid | 3.2 |
| 4 | Maleic acid | 4.0 |
| 5 | Fumaric acid | 5.1 |
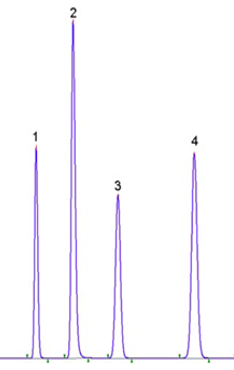
Uracil is highly polar, and it is not retained in the column. Its RT (retention time will considered as t0 and capacity factor of the column will be;
K = (2.2 – 1.5)/1.5 =0.6/1.5 =1.1
The capacity factor (retention factor) is a fundamental concept in HPLC that plays a critical role in assessing and optimising chromatographic separations. By understanding how it is calculated and what factors influence it, analysts can make informed decisions during method development to achieve better resolution, efficiency, and reproducibility. Keeping the capacity factor within an acceptable range ensures that analytes are neither unretained nor excessively retained, leading to more reliable and robust chromatographic performance. Mastering this parameter is essential for anyone involved in analytical method development or routine HPLC analysis.
Related:
References
Abbreviations
Quick Links