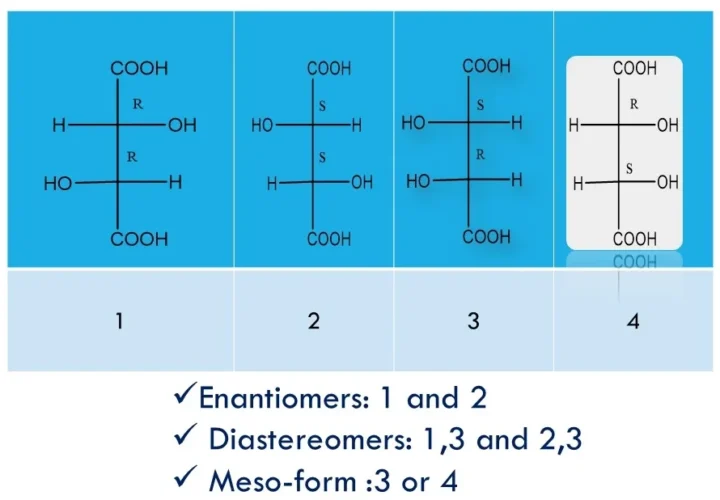
Enantiomers, diastereomers, racemates, and meso-compounds are types of stereoisomers—compounds with the same formula but different 3D arrangements—where enantiomers are non-superimposable mirror images with opposite chirality, diastereomers are non-mirror-image stereoisomers with different properties, racemates are 50:50 mixtures of enantiomers that are optically inactive, and meso-compounds have chiral centres yet are achiral due to internal symmetry. Enantiomers, […]
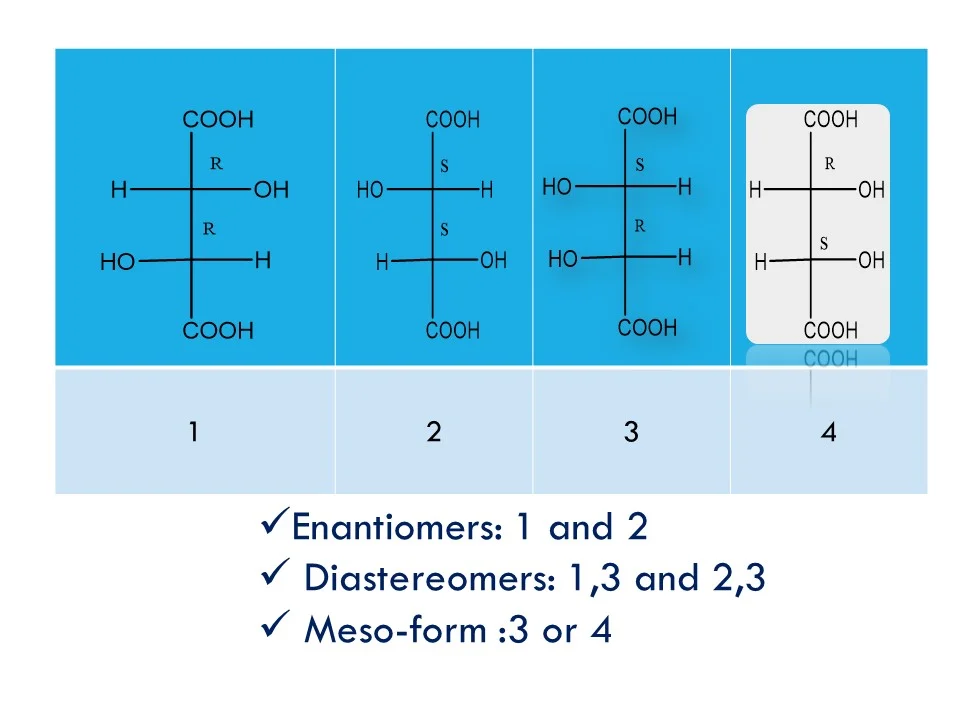
Enantiomers, diastereomers, racemates, and meso-compounds are types of stereoisomers—compounds with the same formula but different 3D arrangements—where enantiomers are non-superimposable mirror images with opposite chirality, diastereomers are non-mirror-image stereoisomers with different properties, racemates are 50:50 mixtures of enantiomers that are optically inactive, and meso-compounds have chiral centres yet are achiral due to internal symmetry.
Molecules with a chiral centre that are non-superimposable on their mirror images are called “Enantiomers”. Enantiomers have the same physical and Chemical properties. d-Lactic acid is the Enantiomer of l-Lactic acid and vice versa.
They have identical physical properties like melting point (mp), boiling point (bp), and solubility, except for optical activity ( rotate plane-polarised light in equal but opposite directions). They can only be separated on a chiral column.
A 1:1 mixture of enantiomers is called a Racemate/Racemic mixture.
This is optically inactive because the rotations of the two enantiomers cancel each other out. They can only be separated on a chiral HPLC or GC
Example: Ibuprofen racemate
Chiral isomers that are not Enantiomers are called Diastereomers. It has different chemical and physical properties. The molecule must have ≥2 chiral centres to show these Diastereomeric properties.
They have different physical and chemical properties, allowing for separation (e.g., by chromatography). They can be separated on an achiral as well as on a chiral column.
Despite the Chiral carbon atom, the molecule is achiral. Chirality is lost by internal compensation.
It possesses an internal plane of symmetry, making its mirror image superimposable on itself. Optically inactive, but unlike a racemate, it’s a single compound, not a mixture.
Stereoisomers include enantiomers (mirror-image, opposite chirality), diastereomers (not mirror images, different properties), racemates (50:50 enantiomer mix, optically inactive), and meso-compounds (chiral centres but achiral due to internal symmetry).
In the above structure:
Boost your pharma career with PharmaGuru’s expert-led online courses.: Online Pharma Course (Training)
| Type | Definition | Mirror Image? | Chirality | Optical Activity |
|---|---|---|---|---|
| Enantiomers | Molecule with chiral centres but internal symmetry | Yes | Chiral | Optically active (equal and opposite) |
| Diastereomers | Stereoisomers that are not mirror images | No | Usually chiral | Typically different optical activities; not equal/opposite |
| Racemate (Racemic Mixture) | 50:50 mixture of two enantiomers | — | Contains equal chiral molecules of both types | Optically inactive due to cancellation |
| Meso-Form | Molecule with chiral centers but internal symmetry | Not applicable | Overall achiral | Optically inactive |
Below is a clear, structured overview of the applications of enantiomers, diastereomers, racemates, and meso-forms in Active Pharmaceutical Ingredients (APIs):
Key role: Many drugs are chiral, and different enantiomers often show different biological activities.
Applications:
Key role: Used in synthesis and purification; diastereomers have different physical properties.
Applications:
Key role: Some APIs are still effectively used in racemic form.
Applications:
Key role: Despite having stereocenters, meso-compounds are achiral.
Applications:
| Type | API Application |
|---|---|
| Enantiomers | Higher potency, reduced toxicity, selective receptor targeting, chiral switches. |
| Diastereomers | Achiral but with chiral centres; simpler regulation; stable intermediates |
| Racemates | Cost-effective production, balanced activity, improved stability, legacy use. |
| Meso-Forms | Achiral but with chiral centers; simpler regulation; stable intermediates |
Together, these stereoisomeric forms (Enantiomers, diastereomers, racemates, and meso-compounds) highlight how subtle changes in three-dimensional arrangement can dramatically influence a molecule’s physical, chemical, and biological behaviour. Understanding their differences is essential for predicting reactivity, optimising drug design, and interpreting stereochemical outcomes in organic chemistry.
Related:
1. To isolate the therapeutically active enantiomer and remove the inactive or toxic one.
2. To improve drug potency, safety, and selectivity in APIs.
3. To meet regulatory requirements for enantiomeric purity.
4. To enable chiral switches—converting racemic drugs into single-enantiomer versions for better clinical performance.
1. A meso compound has chiral centres but is achiral due to an internal plane of symmetry; it is a single molecule and optically inactive.
2. A racemic mixture is a 50:50 mix of two enantiomers; it is optically inactive due to cancellation, not symmetry.
Further reading
Quick Links