Both pKa and pH play a vital role at every stage during drug development. pH measures the acidity of a solution, whereas pKa reflects the intrinsic strength of an acid. pH can vary depending on the concentration of hydrogen ions in a particular solution, while pKa remains a constant characteristic of a specific acid at a […]
Both pKa and pH play a vital role at every stage during drug development. pH measures the acidity of a solution, whereas pKa reflects the intrinsic strength of an acid.
pH can vary depending on the concentration of hydrogen ions in a particular solution, while pKa remains a constant characteristic of a specific acid at a given temperature. The two are linked through the Henderson–Hasselbalch equation, which explains how an acid’s degree of ionisation changes with pH. This relationship shows how pH and pKa together determine whether an acid exists mainly in its protonated or deprotonated form in any chemical system.
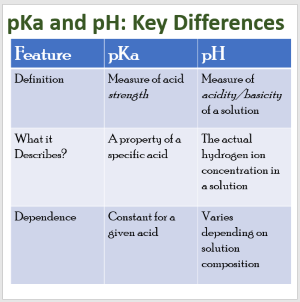
| Feature | pH | pKa |
|---|---|---|
| Definition | A measure of how acidic or basic a solution is. | The acid dissociation constant expressed as –log(Ka); indicates acid strength. |
| What it Represents | The concentration of hydrogen ions (H+) in a solution. | The tendency of an acid to donate a proton. |
| Formula | The intrinsic property of an acid does not change with the amount of acid. | pKa = –log(Ka) |
| What It Depends On | The solution’s actual hydrogen ion concentration. | Changes with dilution, temperature, and additions of acids/bases. |
| Environmental Sensitivity | Determining the acidity/alkalinity of solutions, biology, chemistry, medicine. | Depends mainly on temperature; independent of dilution (for weak acids). |
| Interpretation | Low pH → acidic; High pH → basic. | Low pKa → strong acid; High pKa → weak acid. |
| Scale Range | Generally 0–14 (can be outside in extreme cases). | No fixed range; varies widely depending on the acid. |
| Use Cases | Predicting acid–base equilibrium, buffer design, and reaction mechanisms. | Predicting acid–base equilibrium, buffer design, reaction mechanisms. |
| When They Interact | Predicting acid–base equilibrium, buffer design, and reaction mechanisms. | Helps predict the degree of ionisation at a given pH. |
| Relevance in Buffers | Buffer works best near its pKa value. | Identifies optimal pH for buffer systems. |
| Unit | Dimensionless (log scale). | Dimensionless (log scale). |
Looking to grow your pharma career?
Pick up your course and learn from industry professionals with PharmaGuru’s recognised training programs:
Online Pharma Course (Training)
Understanding the difference helps you:
When pKa = pH, it means the acid is 50% dissociated.
In other words:
This is the point where the acid and its conjugate base are present in equal concentrations.
It also means the buffering capacity of that acid–base system is at its maximum.
The relationship between pH and pKa is described by the Henderson–Hasselbalch equation:
So, pH determines the ionisation state of acids and bases depending on their pKa value.
You May Like
Drug absorption
Buffer preparation
Enzyme function
Protein charge behaviour
Further Reading:
Quick Links