An Uv Visible Spectrophotometer is an analytical instrument that measures the absorbance or transmittance of a sample across the ultraviolet and visible light spectrum (typically 200–800 nm). By passing a beam of light through a sample and detecting the absorbed or transmitted intensity it enables the identification and quantification of substances in liquids or solids. […]
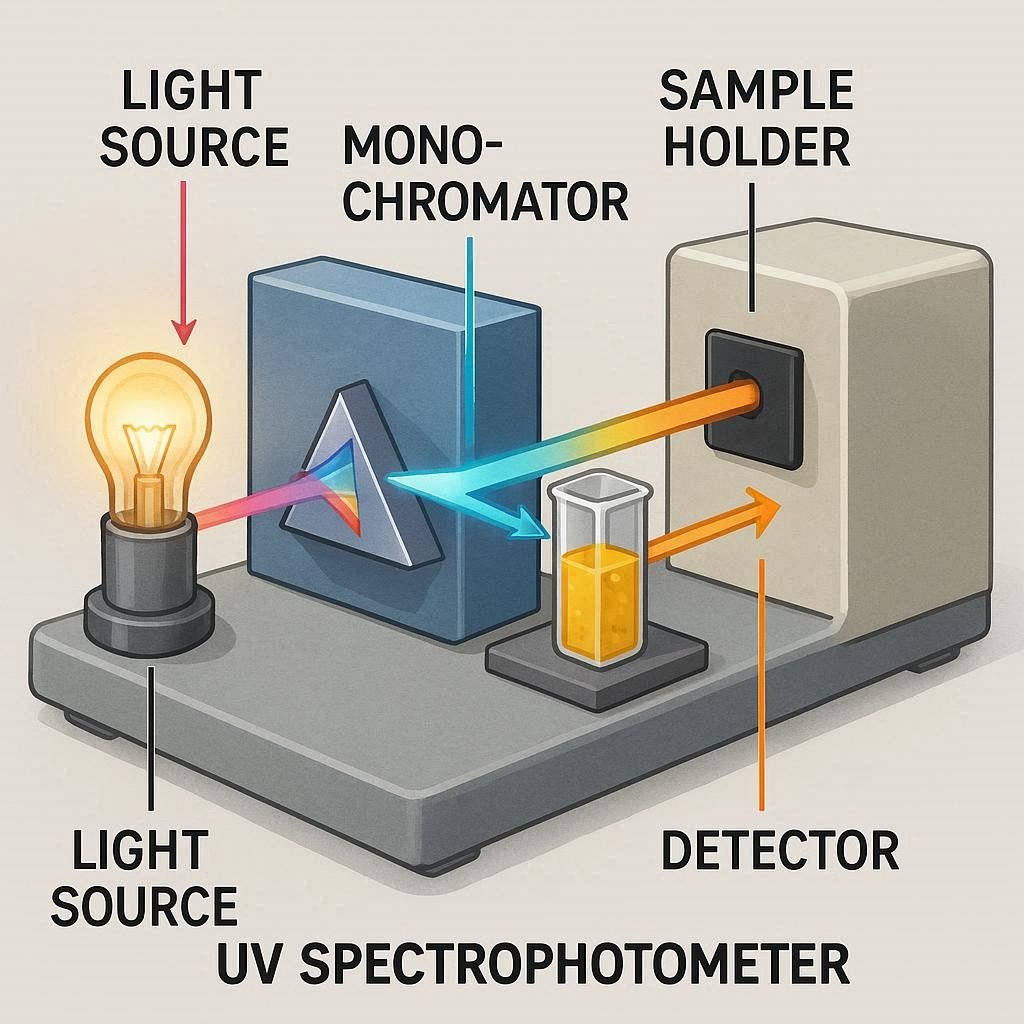
An Uv Visible Spectrophotometer is an analytical instrument that measures the absorbance or transmittance of a sample across the ultraviolet and visible light spectrum (typically 200–800 nm). By passing a beam of light through a sample and detecting the absorbed or transmitted intensity it enables the identification and quantification of substances in liquids or solids. Widely used in chemistry, pharmaceuticals, and biology, it serves both quantitative (e.g., assay, content determination) and qualitative (e.g., identification, wavelength maxima determination) purposes. This article provides a concise, skill-based overview of the principles, applications, and practical uses of UV-Vis spectrophotometry, enhancing understanding through real-world examples and case studies.
The UV/Vis spectrophotometer is one of the most common analytical tools used in the pharmaceutical industry for both assay (quantitative) and identification (qualitative) analysis of drugs. It works by measuring how much ultraviolet or visible light a sample absorbs at specific wavelengths.
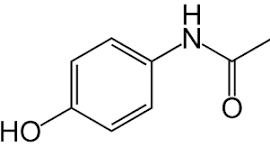
The following is the structure of paracetamol:
Principle
From the structure, it is clear that Paracetamol absorbs ultraviolet light strongly due to the presence of aromatic rings and amide functional groups. According to the Beer–Lambert Law, the absorbance of light is directly proportional to the concentration of the absorbing substance.
Objective: To confirm the identity of paracetamol by determining its characteristic absorption maximum (λmax).
Procedure:
Observation:
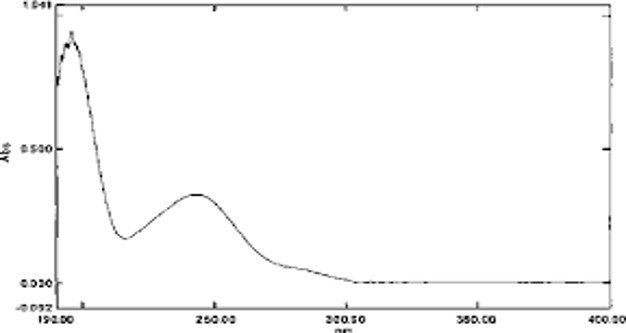
Paracetamol typically shows a λmax around 243 nm in the UV region.
Interpretation:
A peak at ~243 nm confirms the presence of paracetamol since this wavelength corresponds to its characteristic electronic transition.
If a standard is available, both the standard and sample solutions are prepared in the same solvent and scanned from 200–400 nm using a UV/Vis spectrophotometer. If both show a maximum absorbance at approximately 243 nm, the compound is identified as paracetamol.”
Objective: To determine the concentration or percentage purity of paracetamol in a formulation (e.g., tablet).
Procedure:

5. Conclusion
Using a UV/Vis spectrophotometer, both identification and assay of paracetamol can be performed quickly, accurately, and economically.
You May Like
A UV/VIS spectrophotometer is an analytical instrument used to measure the absorbance or transmittance of a sample as a function of wavelength in the ultraviolet (UV) and visible (Vis) regions of the electromagnetic spectrum (typically 190–1100 nm).
It helps determine the concentration, purity, and chemical composition of substances based on their interaction with light.
The UV-visible spectrophotometer works by passing a light beam through a sample to measure the light intensity of a sample. The sample must have UV absorption. The compounds containing functional groups such as C=C, C=O, N=N and aromatic rings are analysed by uv-spectrophotometer
The compounds having UV absorption can be analysed by a UV spectrophotometer such as acetone, toluene, naphthalene, benzoic acid, phenol, paracetamol etc
UV spectroscopy plays an important role in pharmaceutical development due to its simplicity, fast results and cost-effectiveness. It is used for Quantitative analysis, Qualitative analysis and HPLC method development
UV spectroscopy has several limitations such as it is not a specific method, it can not be used for impure compounds and it can not be used for compounds having no UV response.
The instrument operates based on the Beer–Lambert Law, which relates the absorbance (A) of light to the concentration (C) of the absorbing substance and the path length (l) of the sample cell.
When monochromatic light passes through a sample, certain wavelengths are absorbed by the molecules, leading to electronic transitions (usually π → π* or n → π*). The remaining transmitted light is detected and
The Beer–Lambert Law states that absorbance is directly proportional to the concentration and path length:
A=ε×C×l
Where:
A = Absorbance (no units)
ε = Molar absorptivity (L·mol⁻¹·cm⁻¹)
C = Concentration of the solution (mol·L⁻¹)
l = Path length of the cuvette (cm)
Chromophores is a groups of atoms responsible for UV-Visible absorption of the molecules such as C=C, C=O, N=N and aromatic rings.
Redshift is the change in absorption to a longer wavelength
Blueshift is the change in absorption to a shorter wavelength
The solvent used for the sample diluent should not have UV absorption. For example, if any compound has a maximum at 220 nm, then acetone can not be used as a sample diluent. Solvents like water, methanol and ethanol can be used.
FTIR spectroscopy uses a longer, lower-energy wavelength range, typically 4,000 to 400 cm-1 (2,500 to 25,000 nm. UV-Visible spectrophotometers are used between 190 to 900 (UV to visible)
There are mainly two types of UV/VIS Spectrophotometers:
The following are the different Components of a UV Spectrophotometer:
A = log10(I/I0)
where = intensity of incident light and = transmitted light.
UV Spectrophotometer is widely used in the Pharmaceuticals, Biochemistry, Environmental, Food industry and Forensics for various applications such as:
The following are the different applications of UV-visible spectrophotometer:
UV-visible spectrophotometer is used for quantitative analysis such as assay testing, content testing and purity testing of pharmaceuticals
The UV-visible spectrophotometer is used for qualitative analysis such as identification test (by comparing wavelength maxima of standard solution) and purity tests such as transmittance test and absorbance test
The UV-visible spectrophotometer is used to find out the wavelength maxima of pharmaceuticals during the HPLC method development. It is very helpful in deciding the HPLC operating wavelength.
The Absorption Law (combination of Beer’s and Lambert’s laws) states that:
Together, ( A = εcl ).
The UV spectrum plots absorbance vs. wavelength (A vs. λ).A
UV spectrophotometer provides the data in the form of an absorption spectrum. The absorption spectrum is normally presented in the form of a graph, where the y-axis represents the absorbance, and the x-axis represents the wavelengths. Peaks in the spectrum are known as the absorption peaks, indicating the wavelength of light that is absorbed by the sample. The following is the typical representation of the UV spectrum:
Prices vary based on features:
Advantages:
Disadvantages:
Both deuterium and tungsten lamps need to stabilise before accurate measurement.
One beam of light is directed through the sample and then the detector. The same beam is used for both the sample and the reference. The light source, sample, and detector are part of a single optical path. A typical procedure involves measuring the reference first, then switching to the sample, and manually compensating for any variations.
Two beams of light are used: one directed through the sample and the other through a reference (usually air or a blank). A beam splitter divides the light from the light source into two beams – one that passes through the sample and one that passes through the reference. The detectors for each beam are often located side by side, allowing continuous monitoring of both the sample and reference simultaneously. This setup allows for real-time comparison of the sample and reference, providing more accurate and stable measurements.
The following are the main differences between single-beam and double-beam UV-Visible Spectrophotometers:
| Feature | ingle Beam UV-Vis Spectrophotometer | Double Beam UV-Vis Spectrophotometer |
|---|---|---|
| Measurement Method | Two separate beams for the sample and reference | More stable, real-time comparison of the sample and reference |
| Stability | Can be affected by fluctuations in light source and drift | More stable, real-time comparison of sample and reference |
| Precision | Lower precision, manual adjustments required | Higher precision, continuous monitoring |
| Cost | Less expensive | More expensive |
| Complexity | Simpler, fewer components | One beam for both sample and the reference |
Transmittance (T) is calculated by the following formulae:
T=10-ε.c.l
Where: T is the transmittance, ε is the molar absorption coefficient and l is the pathlength
The following is the relationship between transmittance (T) and absorption (A):
A(Absorbance)= -Log T or A =ε.c.l
or Absorbance(A) = (molar absorption coefficient) x (concentration) x (pathlength)
Beer-Lambert law states that the absorbance of a solution is proportional to its concentration. molar absorption coefficient and optical path length.
This equation is very helpful and widely used in the industries by Analytical Scientists. This instrument is used to measure the intensity of the light before and after passes through the sample. This instrument provides reading in terms of transmittance (T) and absorbance (A). Transmittance is the ratio of the intensity to the transmittance light to the intensity of the incident light, express as percentage absorbance. Absorbance is the logarithm of the reciprocal of transmittance and is directly proportional to the absorbing species in the sample.
The Uv visible spectrometertechnique plays a vital role in the pharmaceutical industry for qualitative and quantitative analysis. I hope this article has clarified all your doubts and that you can now use it effectively in pharmaceutical development.
Abbreviations
Further Reading:
Quick Links