Essential HPLC equations and terminology encompass key parameters related to column separation, mobile and stationary phases, flow rate, retention time, and resolution. A clear understanding of these concepts is vital for accurate method development, optimisation, and reliable HPLC analysis. In this blog, I will discuss the fundamental HPLC terms and formulas that every chromatographer should […]
Essential HPLC equations and terminology encompass key parameters related to column separation, mobile and stationary phases, flow rate, retention time, and resolution. A clear understanding of these concepts is vital for accurate method development, optimisation, and reliable HPLC analysis.
In this blog, I will discuss the fundamental HPLC terms and formulas that every chromatographer should know, with explanations rooted in practical applications.
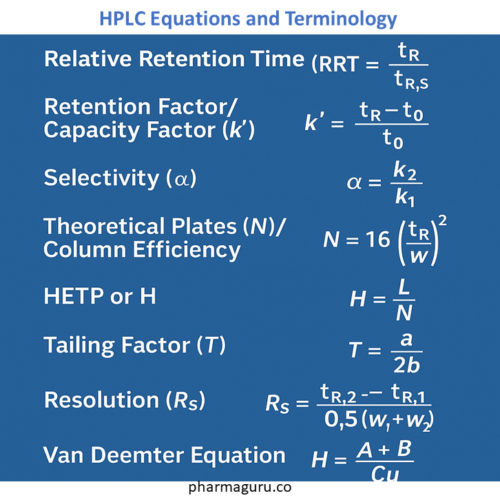
Where:
A chromatogram is the visual output of an HPLC analysis. It displays peaks—each representing a compound in your sample—on a graph where the x-axis is time (usually in minutes) and the y-axis is signal intensity (usually detector response).

Each peak gives valuable information about a compound’s identity, concentration, and behaviour in the column.
The retention time is the amount of time it takes for an analyte to travel through the column and reach the detector. It’s a key factor in identifying compounds, assuming consistent system conditions.
This is the ratio of the retention time of a compound to that of a reference compound.
Equation: see table-1
RRT is useful when comparing chromatograms across different systems or methods, especially in regulatory submissions.
This describes how long a compound is retained compared to an unretained species.
Equation: See table-1
Expert Tip: An optimal k′ value is typically between 1 and 10.
Selectivity is a measure of how well two analytes can be separated based on their retention.
Equation: See Table -1
A higher α indicates better separation potential.
Theoretical Plates are a measure of how efficiently a column can separate compounds. More plates = sharper peaks.
Equation:
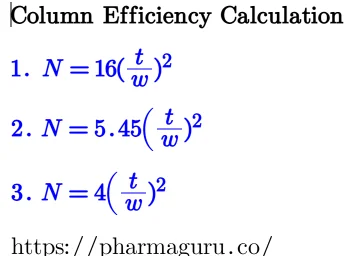
This represents the column length divided by the number of theoretical plates.
Equation: H=L/N
Where L = column length and N is the theoretical plate
Expert Tip: A lower HETP value indicates better column performance.
Resolution tells you how well two peaks are separated.
Equation: See Table 1
Expert Tips:
Equation: See Table 1
Ideal T value = 1.0. Values >1 indicate tailing.
Equation: See table 1
Expert Tip: For ideal peak, A = 1.0
Example: Start with 10% acetonitrile, increase to 90% over 10 minutes.
Both are reversed-phase columns, but differ in hydrophobicity.
| Term | Definition/Use |
|---|---|
| Chromatogram | Visual output of HPLC analysis |
| Retention Time | Time a compound takes to elute |
| Relative Retention Time | Retention time ratio to a reference compound |
| Area Response | Integral under the peak—related to concentration |
| Retention Factor (k′) | How long an analyte is retained vs. dead time |
| Selectivity (α) | Separation power between two analytes |
| Resolution (R) | Peak separation quality |
| Theoretical Plates (N) | Column efficiency indicator |
| HETP (H) | Solvent(s) move analytes through the column |
| Tailing/Asymmetry Factor | Indicates peak distortion |
| Mobile Phase | Solvent(s) moving analytes through the column |
| Stationary Phase | Coated material inside the column |
| Gradient Elution | Changing solvent composition over time |
| C18 / C8 | Common reversed-phase stationary phases |
Understanding these core HPLC terms and equations isn’t just about passing exams or meeting regulatory requirements—it’s about becoming a smarter, faster, and more confident chromatographer.
Whether you’re troubleshooting a distorted peak, optimising a separation, or validating a method, these concepts guide every step of your analysis.
HPLC terminology refers to the key terms and concepts used to describe the components, processes, and performance of High-Performance Liquid Chromatography. This includes terms such as:
1. Mobile Phase – the solvent that carries the analytes through the column
2. Stationary Phase – the material inside the column that interacts with analytes
3. Retention Time – time taken for a compound to elute
4. Flow Rate – speed at which the mobile phase moves through the system
5. Resolution – how well two compounds are separated
6. Retention Factor (k′) – indicates how long an analyte is retained
7. Selectivity (α.) – the separation factor between two analytes
8. Theoretical Plates (N) – a measure of column efficiency
9. Peak Symmetry – describes the shape of chromatographic peaks
These terms help in understanding and controlling chromatographic performance for accurate and reproducible analysis.
Further reading
Quick Links