Evaluation of a pharmacopoeial monograph involves systematically assessing whether a drug substance or product complies with the quality standards outlined in the relevant pharmacopoeia (e.g., USP, EP, BP). This process ensures that marketed medicines meet established criteria for identity, strength, quality, and purity, safeguarding public health. Evaluation typically includes verifying the product’s conformity with the […]
Evaluation of a pharmacopoeial monograph involves systematically assessing whether a drug substance or product complies with the quality standards outlined in the relevant pharmacopoeia (e.g., USP, EP, BP). This process ensures that marketed medicines meet established criteria for identity, strength, quality, and purity, safeguarding public health. Evaluation typically includes verifying the product’s conformity with the monograph’s specifications for chemical, physical, and, where applicable, biological characteristics. It also involves assessing the suitability of analytical methods and ensuring the product’s safety, efficacy, and regulatory compliance
Pharmacopeial monographs are the cornerstone of pharmaceutical quality control. These documents define the identity, strength, purity, and performance of drugs and their ingredients. Whether you’re working in quality assurance, regulatory affairs, or pharmaceutical analysis, knowing how to evaluate a pharmacopeial monograph is essential.
In this blog post, I will discuss a practical approach to monograph evaluation, including what to look for, how to assess compliance, and when to recommend updates.
Major takeaway: FAQs
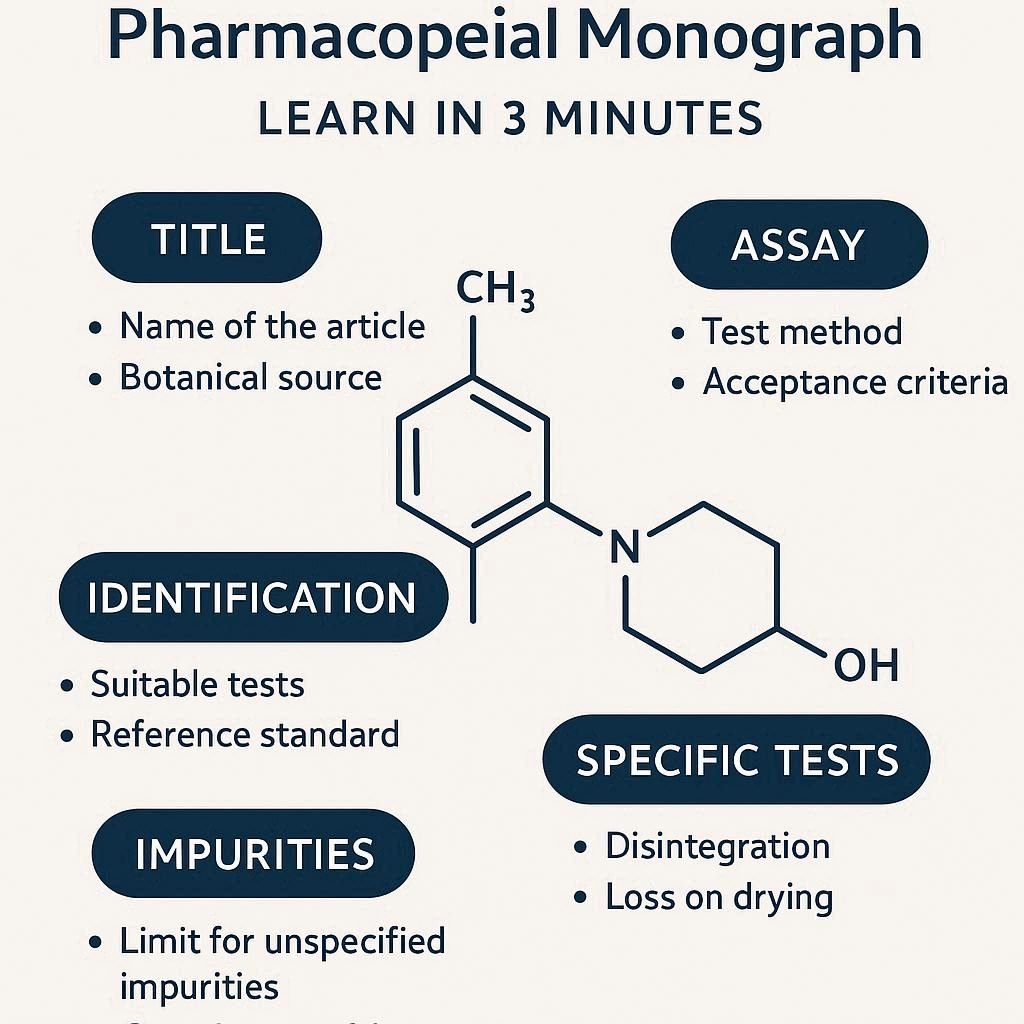
A pharmacopeial monograph is an official standard published by a recognised pharmacopoeia (such as USP, EP, BP, or JP) that outlines the specifications, tests, and analytical methods for a pharmaceutical substance or dosage form.
Pharmacopoeial testing methods include a range of chemical, physical, and biological techniques to verify drug quality. Common methods are:
1. Identification tests (e.g., spectroscopy, chromatography) to confirm the drug’s identity
2. Assay methods (e.g., titration, HPLC) to determine the drug’s strength or potency
3. Impurity and purity tests (e.g., TLC, GC, HPLC) to detect and quantify contaminants
4. Physical tests (e.g., dissolution, hardness, pH, melting point) to assess physical properties
5. Microbiological tests (e.g., sterility, microbial limit tests) for biological safety
6. Other specialized tests such as loss on drying, residue on ignition, and particle size analysis
These standardised methods ensure consistent and reliable evaluation of pharmaceutical quality.
A pharmacopeial monograph is an official standard published by a recognized pharmacopeia (such as USP, EP, BP, or JP) that outlines the specifications, tests, and analytical methods for a pharmaceutical substance or dosage form.
Each monograph typically includes:
You May Like
Evaluating a monograph ensures:
Evaluation is especially important when:
Evaluation of Pharmacopoeia monograph involves the following 7 steps:
1. Verify Identity and Scope
2. Review Specifications and Limits
3. Evaluate Analytical Methods
4. Check Reference Standards
5. Assess Consistency with Global Pharmacopoeias
7. Document Findings and Recommend Revisions
To support your evaluation, consider using:
A pharmacopeial monograph is more than a compliance document — it’s a quality blueprint. Regular and thorough evaluation ensures pharmaceutical products are not only compliant but also safe and effective for patients.
Whether you’re evaluating a monograph for internal QA purposes or preparing a regulatory submission, take the time to assess each element critically. Science, after all, evolves — and our standards must evolve with it.
Further Reading
Quick Links