Sulphated Ash Test In Pharmaceutical Analysis: Why, How, And At What level , procedure, calculation, case studies and FAqs
Sulphated Ash Test In Pharmaceutical Analysis: The Sulphated Ash Test (also known as Residue on Ignition Test) is a quality control procedure used in pharmaceutical analysis to determine the amount of inorganic residue remaining after a sample is incinerated in the presence of sulfuric acid. This residue typically consists of metal salts and other inorganic impurities
The sulphated ash test is a pharmaceutical analysis technique used to quantify the amount of inorganic impurities present in a sample. Primarily applied in quality control, the test involves incinerating the sample in the presence of concentrated sulfuric acid, which facilitates the complete oxidation of organic matter into volatile gases. The remaining non-volatile residue, composed mainly of inorganic salts such as metal sulfates, is then weighed. This method plays a critical role in ensuring the purity and quality of pharmaceutical substances by detecting potential contaminants introduced during manufacturing, handling, or storage.
You may like:
The Sulphated Ash Test is performed to
The sample is ignited at a high temperature in the presence of concentrated sulfuric acid, which helps to convert inorganic materials into stable sulfates. The remaining residue is weighed, and its amount (as a percentage of the original sample) is calculated.
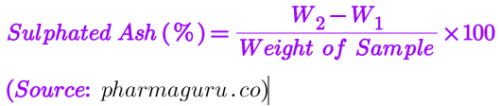
The result must comply with the pharmacopeial limit, often not more than 0.1–0.2%, depending on the substance.
To determine the percentage of sulphated ash in a given Active pharmaceutical ingredient (API):
Sample Details:
Calculation:
Residue weight=W2−W1=30.276−30.254=0.022g
% of Sulphated Ash (Using formula -1) = (0.022/2.00) x 100 = 1.1%
Note: If the limit is NMT (Not More Than) 0.1%, then the API fails in the test
| Aspect | Sulphated Ash | Residue on Ignition (ROI) |
| Definition | The residue remaining after the sample is incinerated with concentrated sulfuric acid. | The residue remaining after the sample is incinerated without any added acid. |
| Use of Sulfuric Acid | Yes. Sulfuric acid is added to convert metals to stable sulfates and ensure complete oxidation. | No. Sample is ignited directly, sometimes with gentle heating first. |
| Purpose | pecifically used to detect and quantify inorganic impurities by converting them into stable sulfates. | More general test to determine total non-volatile residue, including both organic and inorganic residues. |
| Residue Composition | Mostly metal sulfates and other inorganic salts. | May include carbonaceous material if oxidation is incomplete. |
| Accuracy & Completeness | More accurate and specific for detecting metallic impurities. | May be less complete in combustion, especially with organic-rich samples. |
| Pharmacopeial Use | Common in Indian Pharmacopoeia (IP), British Pharmacopoeia (BP), European Pharmacopoeia (Ph. Eur..), etc. | Common in United States Pharmacopeia (USP). |
| Typical Application | Used for substances that may contain metal impurities or require full oxidation. | Used for general quality control where full oxidation is not critical. |
Note:
The sulphated ash content of the tested pharmaceutical sample was found to be 1.10% w/w, calculated based on the residue remaining after ignition in the presence of sulfuric acid. This result provides a quantitative estimate of the total inorganic impurities present in the sample, which may originate from manufacturing residues, excipients, or contamination.
Upon comparing the result with the applicable pharmacopeial limit, it was observed that:
This test is crucial in ensuring the purity, safety, and quality of pharmaceutical substances. Results above acceptable limits necessitate further investigation and possibly corrective actions in the manufacturing or sourcing process.
Related:
The purpose of the sulphated ash test is to quantify the amount of inorganic impurities present in a pharmaceutical
Related Topics:
Reference:
Quick Links