
The Relative Response Factor (RRF) is defined as the ratio between the Response Factor of the impurity and the Response Factor of the main analyte standard. In pharmaceutical analysis, ensuring the quality and purity of Active Pharmaceutical Ingredients (APIs) is of paramount importance. This quality is often determined by analysing the impurity profile, which requires […]
The Relative Response Factor (RRF) is defined as the ratio between the Response Factor of the impurity and the Response Factor of the main analyte standard.
In pharmaceutical analysis, ensuring the quality and purity of Active Pharmaceutical Ingredients (APIs) is of paramount importance. This quality is often determined by analysing the impurity profile, which requires precise testing methods. However, managing and using impurity standards for every possible contaminant during routine analysis can be costly, time-consuming, and impractical for pharmaceutical companies. So, what is the solution?
The answer lies in the Relative Response Factor (RRF), a crucial tool that allows for the effective calculation of impurities without needing to reference every individual impurity standard. This approach not only saves costs and time but also enhances the reliability and efficiency of pharmaceutical testing.
In this blog post, we will dive into everything you need to know about RRF, its significance in High-Performance Liquid Chromatography (HPLC) and Gas Chromatography (GC) analysis, and how it can be applied in impurity testing. We’ll explore topics such as:
By the end of this post, you’ll have a solid understanding of RRF and its vital role in pharmaceutical quality control. Whether you’re new to this concept or looking to refine your skills, this guide will help clarify all your questions and provide valuable insights into how to leverage RRF for more efficient and accurate pharmaceutical analysis.
Related Video:
The Relative Response Factor (RRF) is defined as the ratio between the Response Factor of the impurity and the Response Factor of the main analyte standard. It can also be defined as
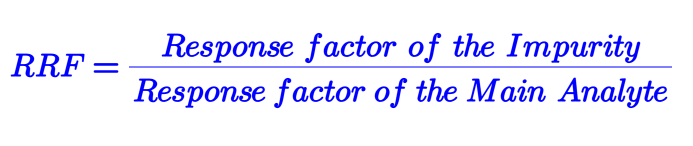
It can also be written as: RRF is the ratio of the slope of the specified impurities to the slope of the main analyte

RRF is used when comparing the responses of different analytes to a standard. It is useful for calculating the concentration of an impurity in a sample when the impurity and the standard have different detector responses.
Note: See Figure 2 to know more about the Response factor
The Relative response factor (RRF) is the ratio of the responses of equal amounts of the Impurities and the drug substance. USP refers RRF as Correction factor (CF) or Response factor or Relative response factor. It is denoted as F
Response Factor, expresses the sensitivity of a detector for a given substance relative to a standard substance. The correction factor is reciprocal of the response factor. Ph.Eur refers RRF as Correction factor or Response factor.
The Response Factor is a relative term, being the response of equal weights of one substance relative to that of another in the conditions described in the test. BP refers RRF as Response factor.
The Response Factor is a measure of the sensitivity of a detector to a specific analyte (compound) relative to its concentration. It is calculated by dividing the detector’s response (area response ) by the amount or concentration of the analyte.
RF helps in quantifying the amount of a specific analyte based on the detector’s response. It is generally used when analysing a single compound and determining its concentration in a sample.

The following are the main differences between RF and RRF
The following techniques are widely used to calculate the impurities in the impurity profile or related substances test:
The following formula is used to calculate the value of impurity:
% Impurity = (Area of impurity in the sample chromatogram÷Sum of area of all peaks in the chromatogram)x100
In this method diluted Analyte standard is used to calculate the impurities
In this method, each impurity is calculated against its corresponding impurity standard.
In Figure-3, sample analyte A contain two impurities B and C. Now let us calculate each impurity B and C using all the above three techniques:
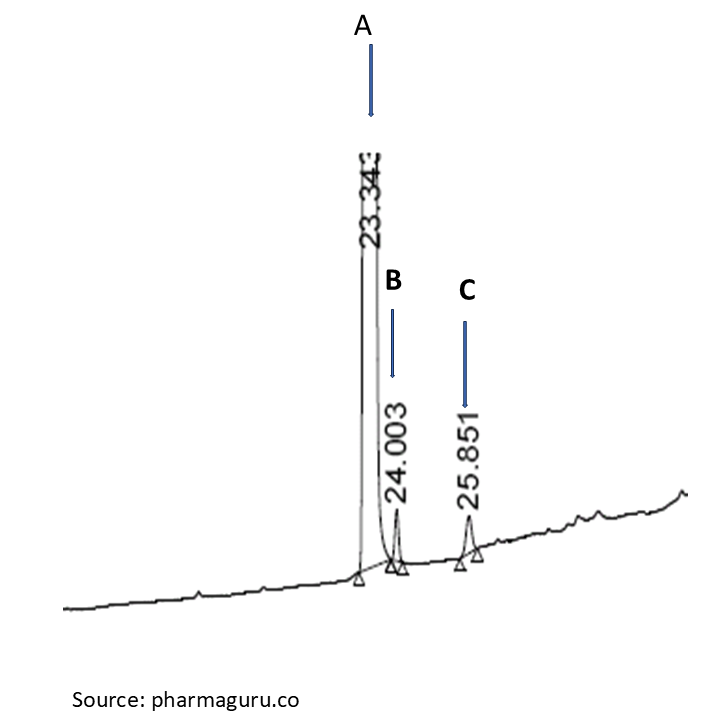
| Peak | Area | % value using Area normalisation/% area | % value using diluted Analyte | % value using external standard |
| A (analyte) | 9878863 | 97.98 | – | – |
| B (Imp) | 155245 | 1.54 | 1.49 | 0.62 |
| C (Imp) | 48695 | 0.48 | 0.44 | 0.41 |
| Total | 100280 | – | – | – |
It is clear from the above (Figure -3 and table-1) that the results obtained by the area normalization method and the diluted standard methods are almost identical for impurity “C”, while the results obtained by the external standard method are different for impurity B.
Now the question is which method is correct out of area normalisation, dilute analyte method and external standard method. In other words, which of the above results is correct?
The answer is that the result obtained by the external standard is correct using all detectors. The result obtained by the Area normalisation method and diluted analyte method can only be correct with universal detectors like ELSD, LC-MS. GC-Ms and not by the UV detector.
As each impurity may have different wavelength maxima and hence, the result obtained by the Area normalisation method and the diluted analyte method is not correct.
The result obtained by the external standard method is correct.
But now the question is, can an external standard method be possible to use every time?
The answer is no because of the following reasons:
Now the question is what is the solution?
To get rid of the above challenges related to calculation by different methods, the Relative Response Factor (RRF) has been introduced. It is defined as the ratio between the Response Factor of impurity and the Response Factor of the main analyte standard :
RRF= Response factor of Impurity/Response factor of the Main analyte
RRF approach is very helpful and acceptable by all regulatory agencies:
The following formula is used to calculate impurity using RRF:

The following methods are widely used in the pharmaceutical industries in calculating the RRF:
Note: Methods 1 and 2 are widely used in the industry for RRF calculation
Steps for Calculation:
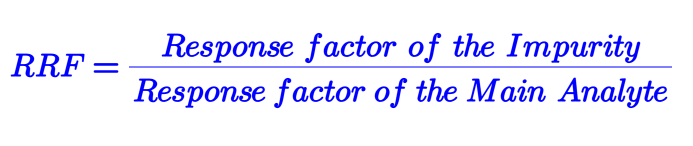
Let us consider we have to determine the RRF of impurity B against drug substance A:
Thus, RRF impurity B is 0.25 against Drug substance A .
The relative response factor will be determined by dividing the slope of specified impurities by the slope of the reference substance.

Let us consider an API which contains impurity “A” with a limit of NMT 0.075%. QL of the method is 0.0075%. The sample analysis concentration is 3000mcg/ml (3mg/ml).
Procedure for RRF of impurity A
Impurity A and API solutions preparation
Note: Similarly API solution can also be prepared
(Table-2)
| % Concentration | API Concentration (mcg/ml) | API AREA | A Concentration (mcg/ml) | A AREA |
| QL | 0.17 | 5030 | 0.17 | 31555 |
| 25 | 0.56 | 12360 | 0.56 | 8275 |
| 50 | 1.125 | 22960 | 1.125 | 16849 |
| 75 | 1.69 | 43941 | 1.69 | 33055 |
| 125 | 2.81 | 55422 | 2.81 | 42070 |
| 150 | 3.38 | 66390 | 3.38 | 50140 |
| Slope | 19224.64333 | NA | NA | 9675.245121 |
| RRF of A | (9675.245121/19224.64333) = 0.50 | NA | NA | NA |
RRF of A = Slope of A /Slope of B = (9675.245121/19224.64333) = 0.50
RRF for A is 0.54, and this will be used while calculating this impurity in routine analysis.
Let us consider an API which contains impurity “I” with a limit of NMT 0.15%. QL of the method is 0.04%. The sample analysis concentration is 400mcg/ml (See figure – )
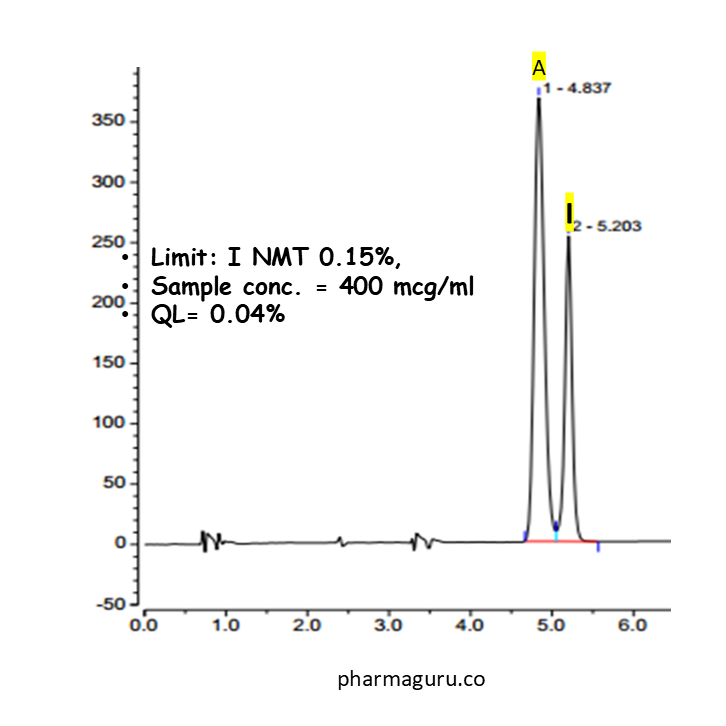
Now, let us discuss the procedure for calculating the RRF of impurity “I“.
Prepare at least 5 concentrations from two stock solutions for each I, as well as for the A at the concentration range QL (quantitation limit) to ≥ 150%, and calculate RRF (See below table -3)
(Table 3)
| “A” Concertation | “A” Response | “I” Concertation | “I” Response |
| 0.13 | 2514 | 0.12 | 1105 |
| 0.29 | 6179 | 0.28 | 2705 |
| 0.60 | 11482 | 0.58 | 5666 |
| 1.15 | 21969 | 1.16 | 11161 |
| 1.44 | 27709 | 1.45 | 14165 |
| 1.76 | 264741.1 | 1.77 | 16959 |
| Slope | 18788.31 | – | 9649.206 |
| RRF of I | 9649.206/18788.31 = 0.514 | – | – |
RRF for I is 0.514, and it will be used while calculating this impurity in routine analysis.
This method requires preparing a series of standard solutions with known concentrations of the impurity and main analyte. The response factors are determined using these standards. It is similar to the direct method but uses multiple data points to improve accuracy.
This is a less common method, and it is widely used in GC analysis. In the standard addition method, a known amount of reference compound is added to the sample, and the response is measured. The RRF is determined by comparing the response from the sample and the added reference.
Relative response factor or RRF may change due to a change in:
RRF of Isomeric impurities may or may not be the same.
Case study : ortho Benzaldehyde, meta hydroxy Benzaldehyde and para hydroxy Benzaldehyde are not identical, RRF or RRF equal to 1
If an unspecified impurity is known, then RRF must be calculated and considered in the impurity calculation. IF the unspecified impurity is unknown, then RRF can not be calculated.
Note: RRF round off rage may be modified (between 0.9 to 1.1 or between 0.95 to 1.05) depending upon the nature of the impurity and process requirement.
The Relative Response Factor (RRF) is crucial in pharmaceutical analysis to ensure accurate and consistent quantification of impurities in complex pharmaceuticals containing multiple impurities. Its importance can be outlined in several key aspects:
In conclusion, the Relative Response Factor (RRF) is an essential parameter in chromatographic techniques, particularly when calculating impurities in pharmaceutical analysis. A thorough understanding of RRF is crucial for the effective development of analytical methods and ensuring the accuracy of routine analyses. By grasping the role of RRF, analysts can better compare the responses of different compounds, leading to more reliable impurity quantification. I hope this article has provided you with a clear and comprehensive understanding of RRF, and you can now apply this knowledge in your chromatographic method development and routine testing.
If you have any further questions or need clarification on any aspect of this topic, please feel free to leave a comment, and I will address your queries as a priority.
Related:
RRF between 0.8 and 1.2 should be considered as 1.0 and may not be used for calculation
The RRF of less than 0.2 or more than 5 should not be used calculation
Relative response factor (RRF) is used to control the impurities by chromatographic methods like HPLC and GC. In this method impurity standard is not used during the analysis, and in place of impurity a RRF is used in the calculation.
There are several methods for calculating the response factor (RRF), of which the slope method is widely used in industry. To calculate the RRF by this method, the slope of the impurity is divided by the slope of the main analyte.
RRF = Slope of an impurity/Slope of the API
Relative response factor (RRF) is used to control the impurities by chromatographic methods like HPLC and GC. In this method impurity standard is not used during the analysis and in place of impurity a RRF is used in the calculation.
Response factor is the 1/RRF
RF is the response factor whereas RRF is the relative response factor.
In the response ratio method, Impurity and Analyte standards are prepared at the same concentration and injected into a chromatographic system to get the chromatograms. The area response of impurity is divided with the area response of main analyte to calculate the response factor.
Different methods like the area normalisation method, external standard method and internal standard methods are used to calculate the impurities. In most of the cases, each method gives different result and that is why RRF method is used for impurity profile calculation.
Response factor = 1/RRF
Different methods like the area normalization method, external standard method and internal standard methods are used to calculate the impurities. In most of the case each method gives different result and that is why RRF method is used for impurity profile calculation.
Several factors like wavelength, buffer concentration, column temperature, column brand and detectors are affecting the RRF.
May or may not be same depending upon the nature of the molecule.
If structure is known in that case can be calculated and if structure is not known in that case can not be calculated.
RRF is the Relative response factor whereas CF is the correlation factor.
CF = 1/RRF
Note:
Quick Links