Pharmaceutical intermediates are chemical building blocks used in the multi-step synthesis of APIs—the active drug components that provide therapeutic effects. Unlike APIs, intermediates are not administered to patients, have no therapeutic effect, and are subject to less stringent regulations. However, their quality directly impacts the safety and efficacy of the final API. Pharmaceutical Intermediates vs […]

Pharmaceutical intermediates are chemical building blocks used in the multi-step synthesis of APIs—the active drug components that provide therapeutic effects. Unlike APIs, intermediates are not administered to patients, have no therapeutic effect, and are subject to less stringent regulations. However, their quality directly impacts the safety and efficacy of the final API.
API (Active Pharmaceutical Ingredient) is the biologically active component in a drug that provides therapeutic effects. Pharmaceutical Intermediates are compounds used in the synthesis of APIs but are not active themselves.
Intermediaries in pharma are chemical compounds or substances used during the production stages of APIs or pharmaceuticals, but are not the final active ingredients. They include precursors or intermediates in synthesis.
Intermediate drugs are compounds used in the production of APIs but are not pharmacologically active. Example: a compound like 4-chloro-3-nitrobenzenesulfonamide is used to produce antibiotics.
Intermediates act as key building blocks in the synthesis of APIs, enabling the gradual transformation of raw materials into effective therapeutic drugs.
Intermediates serve as essential building blocks in the multi-step synthesis of APIs, ensuring the correct structure and function of the final drug.
No, intermediates are less regulated than APIs, but they still need to meet certain quality and safety standards during production.
Yes, impurities or variations in intermediates can impact the purity, efficacy, and safety of the final API and, consequently, the drug.
Raw materials are the starting substances (like basic chemicals) used to produce intermediates, which are then used to synthesise APIs.
Typically, pharmaceutical intermediates are not pharmacologically active and do not act as APIs. However, in rare cases, an intermediate could have medicinal effects, although this is not common.
Pharmaceutical intermediates are used in the production of APIs, assisting in creating the active ingredients that will eventually form the drug product.
Intermediates are typically less pure than APIs, have simpler structures, and are produced at earlier stages of the drug manufacturing process.
These are substances used in the formulation process of pharmaceuticals, such as excipients, binders, or fillers, which help in the final drug production but are not pharmacologically active.
Raw materials are the basic substances used in manufacturing any product, including drugs, while Pharmaceutical Intermediates are specific chemical compounds that are part of the drug synthesis process, usually leading to the final API.
Pharmaceuticals are drugs or medications used to diagnose, treat, or prevent diseases and improve health.
Yes, pharmaceutical intermediates can contribute to impurities in the final API if they are not properly controlled or purified during the manufacturing process. Residual intermediates, by-products, or degradation products may remain in the API, potentially affecting its purity, safety, and efficacy.
To prevent this, strict Good Manufacturing Practices (GMP) and quality control measures are applied during API production to ensure that all intermediates are either completely converted or effectively removed, keeping impurity levels within acceptable regulatory limits.
You May Like
| Aspect | Pharmaceutical Intermediates | Active Pharmaceutical Ingredients (APIs) |
|---|---|---|
| Definition | Compounds that are produced during the synthesis of APIs, typically used as precursors in the manufacturing of APIs. | The active component in a pharmaceutical product that is responsible for the therapeutic effect. |
| Role in Drug Development | Serve as building blocks or intermediates to form APIs. | The key component that provides the intended medical effect in the drug. |
| Chemical Complexity | Often simpler and less complex molecules than APIs. | Typically more complex molecules with a defined pharmacological activity. |
| Stage of Manufacturing | Produced earlier in the drug synthesis process. | Produced later in the drug synthesis process, usually after intermediates are made. |
| Regulatory Oversight | Less regulated compared to APIs, though still subject to quality standards. | Heavily regulated due to its direct effect on human health. |
| Usage | Used in the synthesis or production of APIs or other chemicals. | Directly used in the formulation of the final pharmaceutical product. |
| Purity | Generally lower purity than APIs, as they are not the final product. | Must meet strict purity standards (usually >98%) due to their impact on efficacy and safety. |
| Market/Price | Generally less expensive and sold in bulk to manufacturers. | More expensive due to rigorous production, testing, and regulatory standards. |
| Form | Usually in a raw or unrefined form, before the final API is synthesized. | Refined, active compound that will be incorporated into the drug dosage form (tablets, injectables, etc.). |
| Example | 4-chloro-3-nitrobenzenesulfonamide (a precursor to a drug) | Paracetamol, Ibuprofen, Metformin, etc. |
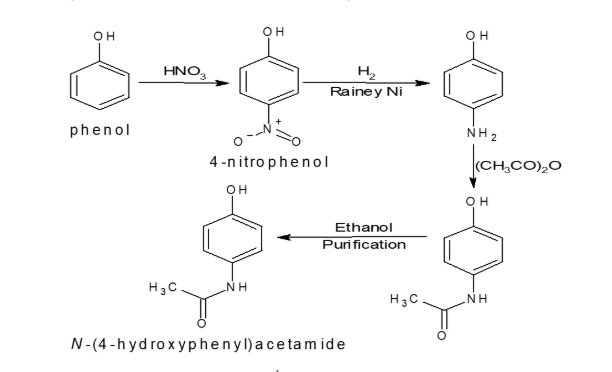
In the above ROS, 4-Nitrophenol and 4-aminophenol are intermediates of API (N-(4-hydroxypheny)acetamide
Further Reading:
Quick Links