HPLC Method Development For Acidic Molecules: A Case Study
HPLC Method Development for Acidic Molecules: In this article, I will discuss the procedure of the stationary phase, mobile phase, buffers and solvents for effective method development of acidic compounds with a case study. After reading this article, you will be able to independently develop an HPLC method for acidic compounds. This article will also enable you to answer several questions:
The following is the structure of Phenol and Benzoic acid.
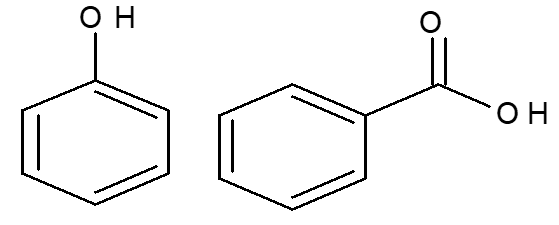
Both compounds have the ability to donate a proton and stabilise themselves due to the resonance effect. Benzoic acid has a greater tendency to donate protons than phenol. Therefore, Benzoic acid is more acidic compared to Phenol.
Those organic compounds tending to donate the H+ ion (proton) and remain energetically stable after the loss of proton are called Acidic Organic Compounds. For example, compounds like Phenol and Benzoic acid are acidic.
Like interact like principle will govern the separation. Here, Molecules are acidic and therefore try to make them less polar/non-polar by suppressing the proton donation ability of the molecule. Keep the mobile phase pH acidic. Then these compounds can be separated in RPC mode using non-polar or moderately polar columns like C18 and C8.
Since Phenol and Benzoic acids are acidic compounds. Therefore, the priority should be to suppress the protonation and make them make them less polar or nonpolar. The only solution is to keep the pH of the Mobile phase acidic ( preferably pH should be less than 4). Phosphate or acetate buffer at concentrations between 0.01 M to 0.03 M and pH less than 4 can be tried for separation. Columns containing moderately polar stationary phases like C8 can be used. C18 columns with less end capping would also be suitable for the separation.
Methanol or Acetonitrile can be used. Considering the cost methanol should be the preferred choice.
These compounds can be separated either in gradient or isocratic mode, but gradient mode should be the preferred choice during method development. The trial should be started with a higher aqueous phase of 78%. Based on the elution of each Benzoic acid and Phenol optimisation in the composition of the mobile phase should be done. Example:
| Time | A (0.02% HCOOH v/v in water pH 3.0) | B (Methanol) |
| 0 | 78 | 22 |
| 15 | 22 | 78 |
| 20 | 22 | 78 |
| 20.1 | 78 | 78 |
| 25 | 78 | 22 |
Keep the flow rate between 0.5 to 1 ml/minute to get column pressure less than 2000 psi
Keep the sample concentration so that there should not be any column overloading, and the peak should be sharp. Sample concentration can be increased or decreased based on requirement e.g sample concentration can be kept like 0.2mg/ml, 0.5mg/ml or 0.7mg/ml
Keep the Injection Volume in such a so that there should not be any column overloading and the peak
should be sharp e.g 5μl, 10μl or 20μl.
Prepare the solution of each Benzoic acid and Phenol and scan in a UV spectrophotometer or PDA detector. Select a wavelength where each Benzoic acid and Phenol have almost equal response.
Inject the standard solution of each Benzoic acid and Phenol and generate the chromatogram. Inject the sample mixture and generate the chromatogram. Based on the elution pattern, optimise the mobile phase composition and chromatographic conditions to get better separation.
Based on the requiremen,t use area % (area normalisation method) or the external standard method to give the result.
Since Benzoic acid is more polar than Phenol. Therefore, Benzoic acid will elute first, and after that Phenol will elute.
In addition to Benzoic acid and Phenol, the above HPLC septation tips can also be applied to other acidic compounds. Now you have learned about column section, mobile phase selection and chromatographic condition optimisation for the separation of acidic compounds, and I hope you can repeat it during method development. That is all about this post. Write your learnings/suggestions related to this post in the comment section.
Related:
Keep the mobile phase pH acidic and use columns like C8 and C18
To separate acidic molecules. Try to make these molecules less polar/non-polar by suppressing their proton donating ability. Keep the mobile phase pH acidic. These compounds can then be separated in RPC mode using non-polar or moderately polar columns such as C18 and C8.
Abbreviations
Quick Links