HPLC method adjustment is necessary to ensure accurate pharmaceutical analysis, since factors such as analytical errors and environmental conditions can cause changes in retention time (RT), relative retention time (RRT), or peak shape. According to the standard test procedure (STP), analysis can not be initiated if the HPLC system fails in the system suitability test. […]
HPLC method adjustment is necessary to ensure accurate pharmaceutical analysis, since factors such as analytical errors and environmental conditions can cause changes in retention time (RT), relative retention time (RRT), or peak shape.
According to the standard test procedure (STP), analysis can not be initiated if the HPLC system fails in the system suitability test. Now the question is, why does the HPLC system fail in the system suitability test, and what is the solution? This is a very critical situation for any analytical or QC professionals. That is why I decided to share my skill-based knowledge on this topic. In this article, I will discuss reasons for failing the system suitability test, the need for adjustment in the HPLC method, necessary and sufficient conditions under which adjustments can be made in the HPLC method, adjustments in the isocratic condition, adjustments in the gradient conditions, case studies and frequently asked questions. After reading this post, all your doubts will be cleared and you will be able to apply more effectively during method development and routine analysis.
HPLC method adjustment is the process of modifying a HPLC method’s operating conditions/parameters to improve its performance and achieve system suitability test (SST) acceptance criteria, such as enhancing resolution, increasing sensitivity (DL/QL), or increasing throughput. It involves making targeted changes to parameters like the mobile phase, flow rate, column, or temperature to optimise the analysis without completely redeveloping the method. Adjustments must often remain within specific limits, especially for validated methods, to ensure they can be performed without a full revalidation
The following factors may lead to failure in the system suitability test and variation in the retention time (RTs), relative retention time (RRTs), co-elution of peaks, and elution pattern:
Hence, HPLC conditions must be adjusted to meet RTs, RRTs , SST criteria and elution pattern.
Boost your pharma career with PharmaGuru’s expert-led online courses.: Online Pharma Course (Training)
Adjustment to the HPLC method is made when it fails in RTs, RRTs, SST criteria and elution patterns. When the specified column is not available as outlined in the method and an equivalent column is used in the analysis, in that case method can also fail in RTs, RRTs, SST criteria and elution patterns. This is why the HPLC condition is adjusted to meet the RTs, RRTs, SST criteria and elution patterns. Since HPLC analysis is performed in isocratic and gradient conditions and therefore adjustments are applicable in both conditions.
Changes or adjustments in the stationary phase are not allowed e.g. C18 stationary phase can not be used in place of the C8 stationary phase and vice-versa.
A change from total porous particle (TPP) to superficially porous particle (SPP) is permitted, provided that the plate number (N) is -25% to 50% relative to the prescribed co
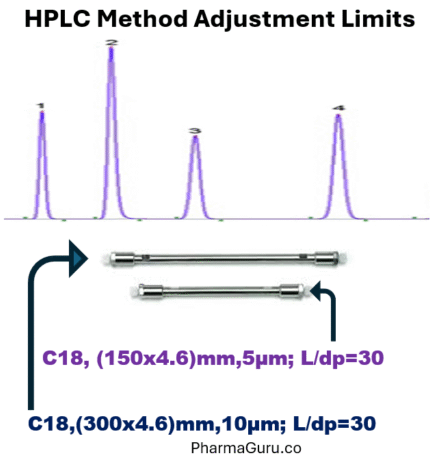
The adjustments in length of the column and particle size are permitted, provided that the ratio of the column length (L) and particle size (dp) remains constant or in the range between -25% to +50% of the prescribed L/dp ratio (L ÷ dp).
Example: if you have a method in which which column is C18,(300 x 4.6) mm, 10µm. Its L/dp will be 30 or L/C =30. Hence, you may use the following C18 columns:
The flow rate of the mobile phase should be adjusted when the column particle size changes or the internal diameter of the column changes. The following equation should be used to adjust the flow rate:
F2 = F1 x [(dc22 x dp1/dc12 x dp2 )] (Equation-1)
Where:
Example-1: The specified ratio of the mobile phase is a mixture of water and methanol: 50 : 50. Hence, the adjustment amount will be calculated in the following way:
30% of 50 is 50 x 30/100 = 15% absolute. But 15% exceeds the maximum permitted change of ±10% absolute. Therefore, the mobile phase ratio may be adjusted only within the range of 40 : 60 to 60 : 40.
Example-2: The specified mobile phase ratio is a mixture of water: methanol: 2 : 98. Hence, the adjustment amount will be calculated in the following way:
30% of 2 is 2 x (30 ÷ 100) =0.6% absolute. This is within the maximum permitted change of ±10% absolute in either component. Therefore, the mobile phase ratio may be adjusted only within the range of 1.4: 98.6 to 2.6 : 97.4.
The specified ratio of the mobile phase is a mixture of Water, Acetonitrile and methanol: 70 : 25 : 5. Hence, the adjustment amount will be calculated in the following way:
Adjustment in acetonitrile: 30% of 25 is 25 x 30/100 = 7.5% absolute. This is within the maximum permitted change of ±10% absolute in either component. Therefore, this component may be adjusted within the range of 32.5% to 17.5% absolute
Adjustment in methanol: 30% of 5 is 5 x 30/100 = 1.5% absolute. This is within the maximum permitted change of ±10% absolute. Therefore, this component may be adjusted within the range of 6.5% to 3.5% absolute.
Please note that in all the cases a sufficient amount of the first component is used to give a total of 100% or in other words, some of all components (water, acetonitrile and methanol) must be 100.
Therefore maximum range will be 62.5 : 32.5 : 5 to 77.5 : 17.5 : 5 or 68.5 : 25 : 6.5 to 71.5 : 25 : 3.5 would meet the requirement
The allowable adjustment of the pH of the aqueous component is ± 0.2 pH units unless otherwise prescribed.
Example: If the specified pH is 7. Then it may be adjusted between 6.8 to 7.2
The allowable adjustment for the buffer concentration is ±10%
Example: If in a method concentration of KH2PO4 is 20mM. Then it will be adjusted between 0.18mM to 0.22mM
An adjustment of the flow rate by ±50% is permitted.
Example: If the flow rate in any method is 1mL/minute. Then it will be adjusted between 0.5mL/minute to 1.5mL/minute
No adjustment is permitted
When the column dimensions are changed , the following equation should be used for adjusting the injection volume:
Vinj2 = Vinj1 (L2dc2 2)/(L1dc1 2)
Where:
This equation will not be applicable to change in stationary phase from TTP columns to SPPs columns
The modified injection volume must pass the following parameters:
Since most of the analysis is performed in gradient mode and therefore adjustments in chromatographic conditions in gradient mode need more precautions than in isocratic mode.
The adjustment in the following chromatographic parameters will be made the same as in adjustment in the LC condition in Isocratic elution
Column volume will be changed due to a change in column dimensions.Since the gradient elution pattern depends upon the column volume and therefore gradient time must be modified. The following equation should be used to calculate the gradient time:
tG2 = tG1 x (F1/F2) [(L2 x dc22 )/L1 x dc12 )]
Where:
The adjustment in conditions for gradient elution requires three steps:
The allowable adjustment in column temperature is ±5oC
Example: If column temperature is 30oC is mentioned in the method. Then the adjustment can be done between 25°C to 35oC during analysis.
The Adjustment in the mobile phase composition and gradient is allowed in the following conditions:
This USP guideline on changes in chromatographic method is invaluable. This guiling is more suitable for isocratic methods compared to the gradient methods. This will be applicable only for the USP monographs. For In-house or method of other methods, revalidation or mini-validation/verification must be performed, and mini-validation must include all allowable changes. This is all about this article. I hope all your doubts have been cleared, and you can apply it more effectively during HPLC method development.
Related:
You May Like
System suitability test may fail due to improper washing of the column, column degradation, error in mobile phase preparation, lab temperature and improper system equilibration
Column length and particle size can be adjusted but L/dp (where L is the length of the column and dp is the particle size of the column) should be between -25 to 50% of the original column. Stationary phase chemistry can not be changed. Flow rate can be adjusted to ±50% of the original flow rate. Keep in mind any adjustment must pass the system suitability test.
Column length and particle size can be adjusted but L/dp (where L is the length of the column and dp is the particle size of the column) should be between -25 to 50% of the original column. Stationary phase chemistry can not be changed. The flow rate can be adjusted to ±50% of the original flow rate. Column temperature can be adjusted to ±5oC and pH can be adjusted to 0.2 pH unit. Keep in mind any adjustment must pass the system suitability test.
No. In-house method changes will be made as per the validation report.
It will be applied to all HPLC monograph methods. It can not be applied in-house or by other methods. Keep in mind that any adjustment must pass the system suitability test.
Yes. Keep in mind that changes must pass the system suitability test
Quick Links