GC method adjustment is often necessary during routine pharmaceutical analysis to achieve acceptable system suitability criteria and prevent failures caused by analytical errors. Analysis can not begin if the GC system fails in the system suitability test as per the standard testing procedure (STP). Now the question is, why does the GC system fail in […]
GC method adjustment is often necessary during routine pharmaceutical analysis to achieve acceptable system suitability criteria and prevent failures caused by analytical errors.
Analysis can not begin if the GC system fails in the system suitability test as per the standard testing procedure (STP). Now the question is, why does the GC system fail in the system-suitability test, and what is its solution? This is a very critical situation for any analytical or QC professionals. That is why I decided to share my skill-based knowledge on this topic. In this article, I will discuss reasons for failing the system suitability test, the need for adjustment in the GC method, the necessary and sufficient conditions under which adjustments can be made in the GC method, case studies and frequently asked questions. This post will clear all your doubts related to GC method adjustment, and your knowledge will increase to the next level.
GC method adjustment is the process of optimising a gas chromatography (GC) method by modifying system parameters to achieve system suitability (SST) criteria and improve separation, sensitivity (DL/QL), and overall analytical efficiency. This may include adjusting GC conditions such as oven temperature programs, carrier gas flow rates, column characteristics (e.g., coating thickness and dimensions), and injection parameters to enhance peak resolution, improve signal quality, and reduce analysis time.
Related: GC Method Validation For Impurities Analysis: How To Get Mastery In 3 Minutes
When GC fails in RTs, RRTs, SST criteria and elution patterns, these failures require adjustment in the GC method . System-suitability test can also fail if equivalent columns are used. That is why the GC condition is adjusted to meet the RTs, RRTs, SST criteria and elution patterns.
The maximum allowable reduction of particle size is 50%. No increase in particle size is allowed.
Note: Now a days packed columns are not used due to its low efficiency.
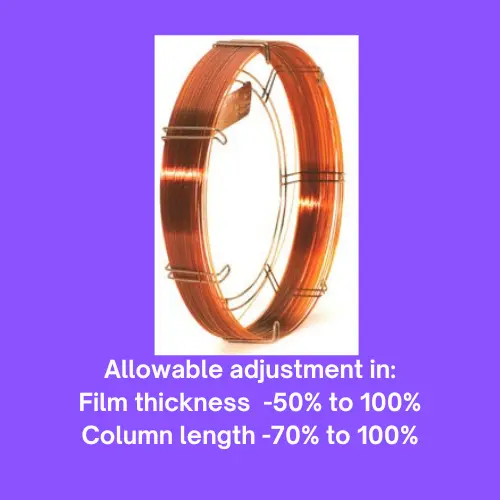
Allowable adjustment in the film thickness is -50% to 100%.
Example: If the film thickness of the USP monograph method is 0.50µm, then the allowable adjustment will be 0.25µ m to 0.75 µm
Allowable adjustment in the Column length is -70% to 100%.
If the column length of the USP monograph method is 50 meters, then the allowable adjustment will be 35 m to 75 m
Allowable adjustment in the Column diameter is ± 50%
If the column internal diameter of the USP monograph method is 300 µm, then the allowable adjustment will be 150µm to 450µm
Allowable adjustment in the Column temperature is ± 10oC
Allowable adjustment of ramp rate and hold time is permitted upto ±20%
Adjustment of the flow rate by ±50% is permitted.
If the flow rate in any method is 10 mL/minute. Then it will be adjusted between 5 mL/minute to 15 mL/minute
Adjustment in injection volume and split ratio adjustment can be made to meet the SST requirement.
Allowable adjustment in injection port temperature and transfer-line temperature ± 10oC, provided no decomposition or condensation occurs
Boost your pharma career with PharmaGuru’s expert-led online courses.: Online Pharma Course (Training)
This USP guideline on adjustment in the GC chromatographic method is very helpful for both quality control and analytical professionals. For In-house or method of other sources revalidation or mini-validation must be performed and mini-validation must include all allowable changes. Now I hope all your doubts have cleared related to this topic and you can apply it more effectively during GC method development and routine analysis.
You May Like:
System suitability test may fail due to improper column conditioning, column degradation, purity of the carrier gas, lab temperature and purity of the solvents
Allowable adjustment in the column length is -70% to 100%, film thickness is -50% to 100%, and flow rate is ±50%.
Allowable adjustment in the column length is -70% to 100%, film thickness is -50% to 100%, and flow rate is ±50%.
It will be applied to all GC monograph methods of the USP. It can not be applied in-house or by other methods. Keep in mind that any adjustment must pass the system suitability test.
es. Keep in mind that changes must pass the system suitability test
Abbreviations
References
Quick Links