Both CROs and CMOs play crucial roles in drug development in the pharmaceutical industry. CROs focus on research activities such as clinical trial design, regulatory support, and data analysis, while CMOs specialise in the large-scale manufacturing of drug substances and finished products. A third type, the CDMO (Contract Development and Manufacturing Organisation), combines both functions—offering […]
Both CROs and CMOs play crucial roles in drug development in the pharmaceutical industry. CROs focus on research activities such as clinical trial design, regulatory support, and data analysis, while CMOs specialise in the large-scale manufacturing of drug substances and finished products.
A third type, the CDMO (Contract Development and Manufacturing Organisation), combines both functions—offering integrated services from early development through to commercial production.
Note:
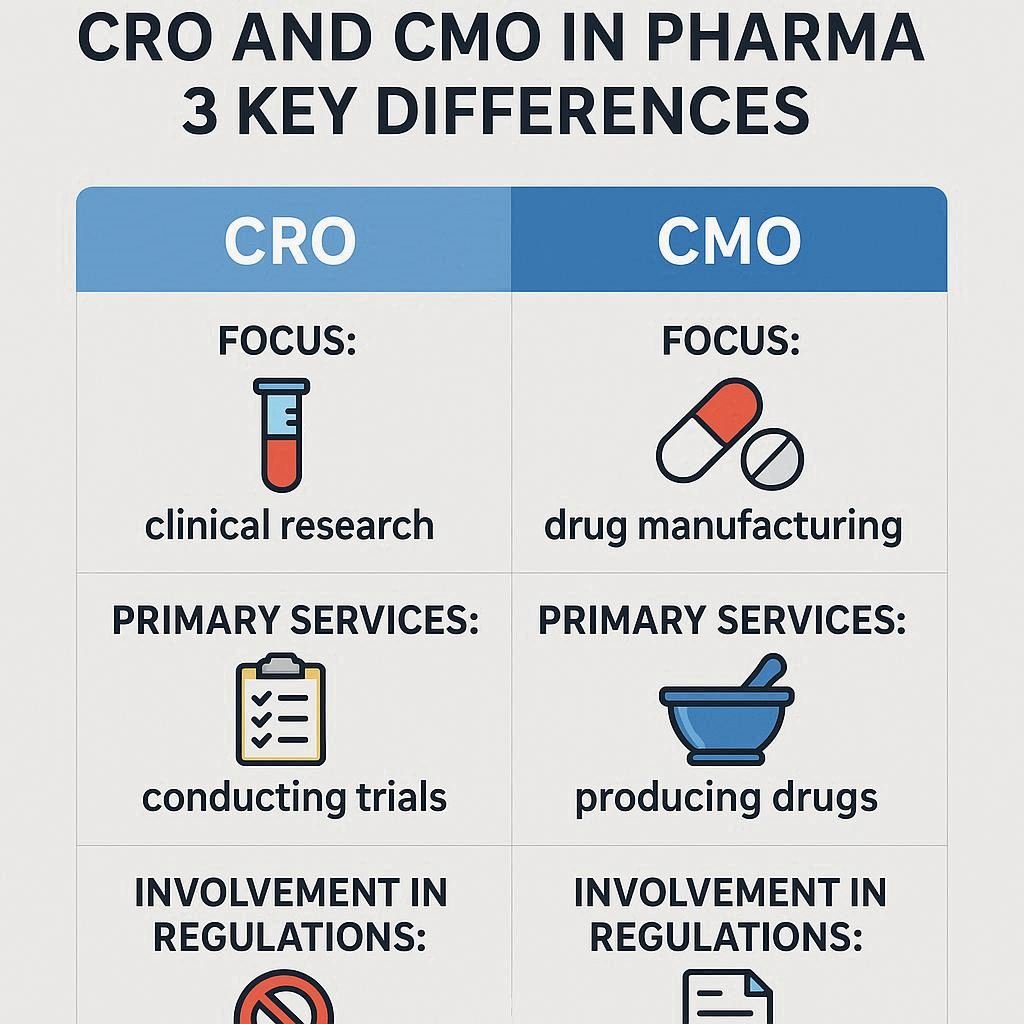
In pharma, a CRO (Contract Research Organisation) handles research and clinical trials, while a CMO (Contract Manufacturing Organisation) manages drug production and manufacturing. They are separate but often work together in the drug development process.
No, a CMO does not report to a CRO. They serve different roles. The CRO oversees research and clinical trials, while the CMO is responsible for manufacturing. Both may report to the sponsoring pharma company but not to each other.
No, a CDMO (Contract Development and Manufacturing Organisation) combines the services of both a CRO and a CMO—offering development, research, and manufacturing. A CRO focuses only on research and clinical trials.
A CMO (Contract Manufacturing Organisation) is a third-party partner that manufactures pharmaceutical products, including active ingredients and finished drugs, often handling packaging and scaling for commercial supply.
A CRO (Contract Research Organisation) is a company that supports pharmaceutical and biotech firms with clinical trial management, regulatory affairs, data analysis, and drug development services.
A CRO handles research and clinical trials, while a CMO handles drug manufacturing. CROs are involved early in development; CMOs come in during later stages for production and distribution.
You May Like
A CRO is a third-party company hired by pharmaceutical or biotechnology firms to support research and development (R&D) efforts, particularly in clinical trials.
CROs allow pharma companies to conduct trials without the burden of building in-house infrastructure, saving both time and cost.
A CMO, on the other hand, is engaged once a drug candidate is ready for production. CMOs provide end-to-end manufacturing services, from small-scale batches for clinical trials to full-scale commercial production.
CMOs help companies bring products to market faster by leveraging specialised facilities and regulatory expertise.
| Aspect | CMO (Contract Manufacturing Organisation) | CMO (Contract Manufacturing Organization) |
|---|---|---|
| Primary Role | Research and clinical development | Drug substance and product manufacturing |
| Focus Area | Preclinical and clinical trial phases | Manufacturing and commercialization |
| Expertise | Clinical operations, regulatory, data | Process engineering, scale-up, compliance |
| Engagement Phase | Early to mid-stage of drug development | Mid to late-stage and commercial supply |
| End Deliverable | Regulatory-ready data and submission docs | Market-ready drug products |
Yes, some organisations are full-service providers known as CDMOs (Contract Development and Manufacturing Organisations), offering both research and manufacturing capabilities. This integrated model can streamline transitions from clinical development to commercial production, reducing handoff risks and timeline delays.
When choosing between a CRO or CMO (or a CDMO), consider the following:
Understanding the distinctions between CROs and CMOs isn’t just a technical exercise—it’s a strategic necessity. Choosing the right partner at the right time can mean the difference between a smooth regulatory approval and costly delays.
By aligning your development phase with the right external expertise, you can bring your product to market faster, with higher quality and lower risk.
Whether you’re just starting your clinical journey or preparing for commercial launch, know your needs, ask the right questions, and choose your partners wisely.
Boost your pharma career with PharmaGuru’s expert-led online courses.: Online Pharma Course (Training)
Quick Links