Key Differences Between SOP and STP: A Comprehensive Guide with 15+ FAQs
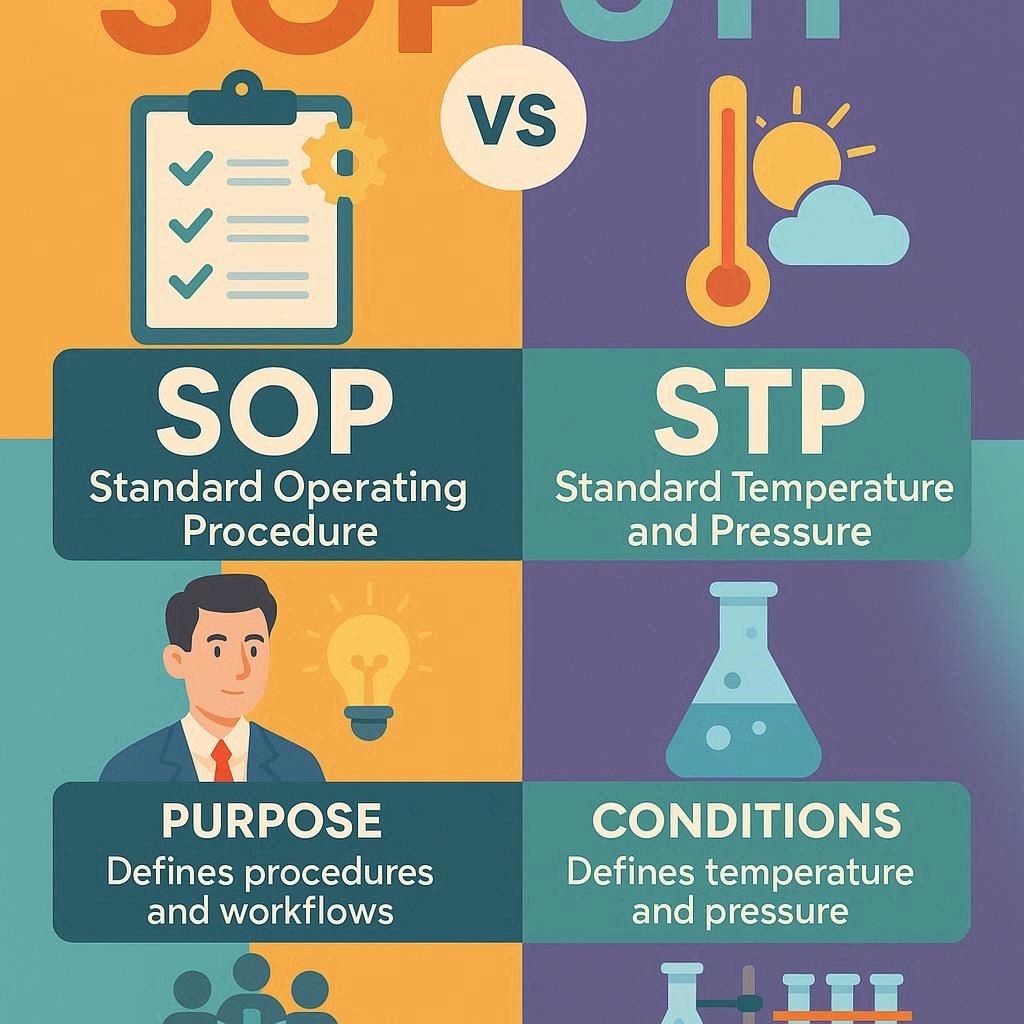
Both SOP and STP play a vital role in maintaining the quality, safety and efficacy of a pharmaceutical in the pharmaceutical industry. An SOP (Standard Operating Procedure) outlines general instructions for routine tasks, ensuring consistency and efficiency. In contrast, STP refers Standard Testing Procedure related to the method of analysis. While the SOP provides the […]
Active Pharmaceutical Ingredients (APIs) Vs Raw Materials: FAQs

Active Pharmaceutical Ingredients (APIs) are the biologically active components of a drug responsible for its therapeutic effect. They are synthesised from raw materials through complex chemical processes. In the final drug product, APIs are combined with excipients—inactive substances that aid in the delivery and stability of the drug but have no therapeutic effect Active Pharmaceutical […]
Melting Point Vs Boiling Point: Interview Questions
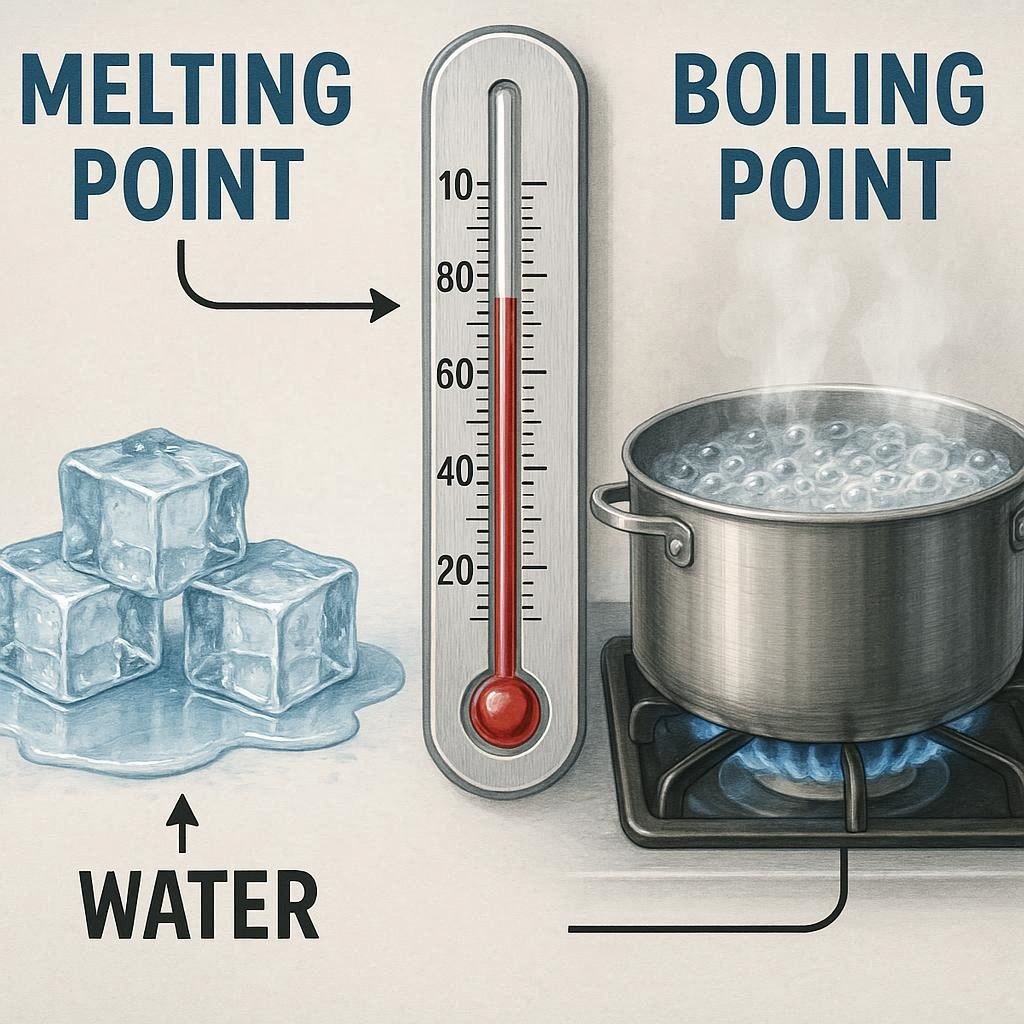
Melting Point Vs Boiling Point Property Melting Point Boiling Point Definition The temperature at which a solid turns into a liquid. The temperature at which a liquid turns into a gas. Phase Change Solid to liquid. Liquid to gas. Energy Required Energy is required to overcome the forces holding the molecules in the solid state. […]
Column Chromatography: Interview Questions
Column Chromatography: Interview Questions What are the different steps involved in column chromatography? The key steps involved in column chromatography are: What-are-the-problems-associated-with-column-chromatography? The following are 12 common problems associated with column chromatography: 1. Sample Overloading 2. Poor Resolution 3. Flow Rate Issues 4. Uneven Packing 5. Solvent Selection 6. Column Contamination 7. Back Pressure 8. […]
The Drug Development Process: 61+Interview Questions
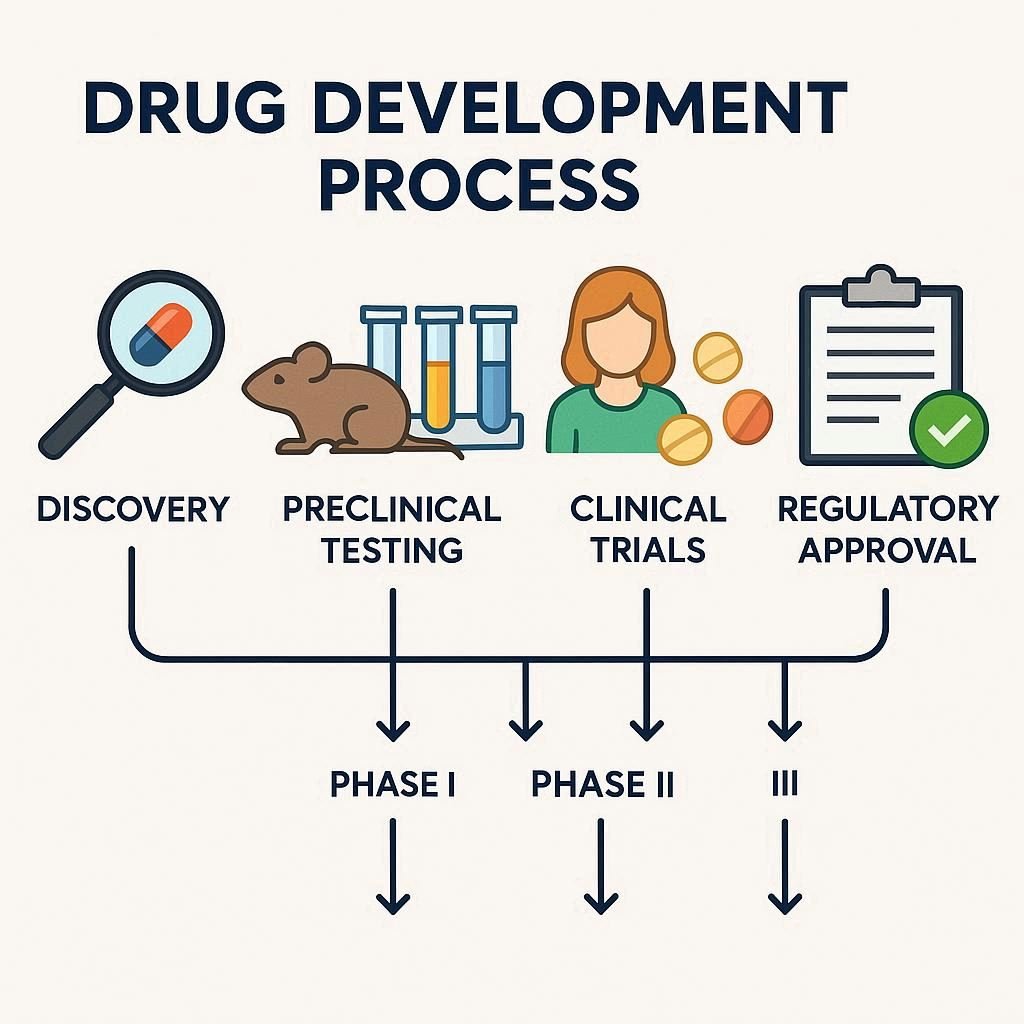
The drug development process is a rigorous, multi-phase journey that includes: Discovery and Preclinical testing to identify and evaluate potential drugs in labs and animals; Clinical Research (Phases 1–3) to assess safety, dosage, and efficacy in humans; FDA Review for regulatory approval based on all collected data; and Post-Market Monitoring to ensure long-term safety and […]
Preparative HPLC Vs Analytical HPLC
Preparative HPLC Vs Analytical HPLC: Key Differences Parameter Preparative HPLC Analytical HPLC Purpose To isolate and purify large quantities of a compound Quality control, method development, and content analysis Sample Size Large (milligrams to grams) Small (micrograms to milligrams) Column Size Larger diameter and longer columns (e.g., 10–50 mm ID) Smaller diameter and shorter columns […]
Control Samples Or Retention Samples or Reference Samples in Pharmaceuticals: Learn With 5+ FAQs
Control Samples Or Retention Samples or Reference Samples are reference materials with known properties used to verify the accuracy and reliability of tests, serving as benchmarks to ensure consistent, acceptable results. These samples play a critical role in post-market surveillance, regulatory compliance, and the investigation of complaints. In this article, I will discuss the definition, […]
What is a Pharmaceutical Technical File And What It Contains: Lean In 5 Minutes
A Pharmaceutical Technical File is a detailed compilation of documents that proves a product’s compliance with applicable regulations and standards. It is a mandatory requirement for many products, especially those requiring CE marking within the European Union. The file serves as crucial evidence of the product’s safety, efficacy, and overall performance. Technical File document acts […]
Lessons Learned Approach in Pharmaceutical Development: Get Mastery with 7+ FAQs
The Lessons Learned approach is a structured process of capturing, analysing, and applying insights from past successes and failures to enhance future projects and prevent the recurrence of mistakes. In the highly regulated and innovation-driven pharmaceutical development, mistakes can be costly, not just financially, but in terms of time, compliance, and patient safety. That’s why […]
What Is Change Control (CC) in Pharmaceutical and Why Is It Essential: Learn In 3 Minutes
Discover the importance of Change Control in pharmaceuticals—its definition, purpose, process, guidelines, and benefits. Learn how it ensures product quality and regulatory compliance.
