Both Assay and Purity play a unique role in controlling quality, safety and efficacy of a pharmaceutical at each stage during drug development. An assay is the quantitative measurement of the active pharmaceutical ingredient in a substance, while purity refers to the assessment of the presence and level of impurities, involving qualitative or quantitative analysis. […]
Both Assay and Purity play a unique role in controlling quality, safety and efficacy of a pharmaceutical at each stage during drug development. An assay is the quantitative measurement of the active pharmaceutical ingredient in a substance, while purity refers to the assessment of the presence and level of impurities, involving qualitative or quantitative analysis.
An assay refers to the quantitative measurement of the active pharmaceutical ingredient (API) in a drug product or raw material. Essentially, it’s a test that tells you how much of the desired compound is actually present.
Example: If a tablet is labelled to contain 500 mg of paracetamol, an assay test will verify whether the tablet contains 500 mg, and how much more or less it might have.
Assay is calculated by the following 1- External standard method and 2- internal standard method:
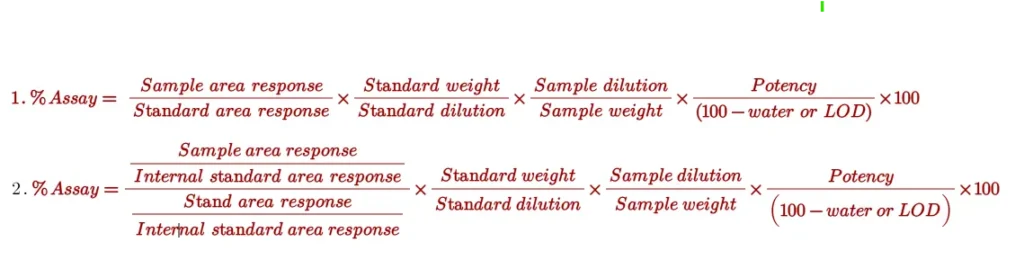
Note: In Industry external standard method is widely used to calculate purity
Purity refers to the qualitative composition of a drug substance or its intermediates. It can be determined using various techniques, including chemical, spectroscopic, and chromatographic methods. While chemical and spectroscopic methods are often less specific and selective, making them less commonly used for purity analysis, chromatographic techniques offer high specificity and selectivity. This is why chromatography is the preferred method for assessing purity in the pharmaceutical industry.
Example: Phenol 98.5% pure; It indicates the tentative qualitative purity of phenol (which is 98.5 area%)
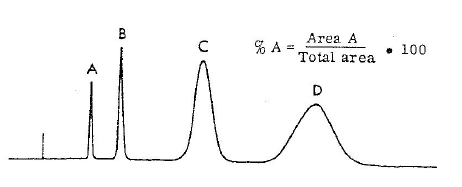
Purity is calculated by area normalisation or area% method by the following formula:
| Purity | Assay |
| It is qualitative content of a drug substance or a molecule | It is the qualitative content of a drug substance or a molecule |
| It is absolute value | It is relative value |
| Purity depends upon the impurities present in the pharmaceuticals | Assay depends upon potency ( while using spectroscopic and chromatographic techniques). |
A drug with high purity but a low assay may not deliver the intended therapeutic effect.
Therefore, both parameters are critical in pharmaceutical quality control and are often tested side-by-side during raw material evaluation, in-process checks, and final product testing.
Example: Benzoic Acid
| Test | Result | Acceptance Criteria | Status |
|---|---|---|---|
| Assay | 97% | 99.0% – 101.0% | Fail |
| Purity | 99.6% | Not applicable | Pass |
In pharmaceutical analysis, assay and purity are not interchangeable. Think of assay as measuring how strong the medicine is, while purity tells you how clean it is. Together, they ensure that what reaches the patient is both effective and safe.
Related
An assay is the quantitative measurement of the active pharmaceutical ingredient in a substance, while purity refers to the assessment of the presence and level of impurities, involving qualitative or quantitative analysis.
Further Reading
Quick Links