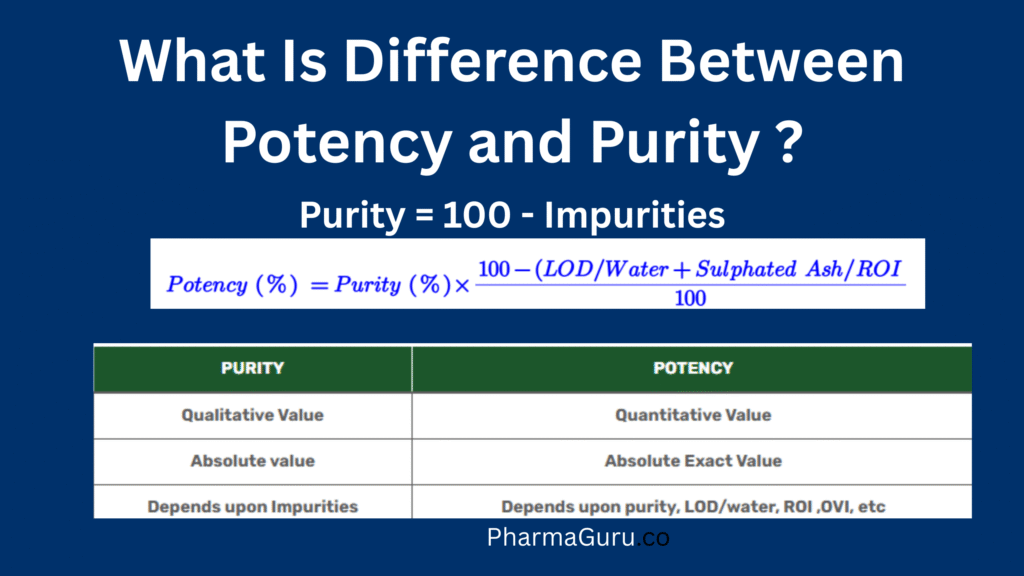
Potency and purity play a vital role in pharmaceutical analysis in managing quality, safety and efficacy of a pharmaceutical. Purity is the qualitative content of any drug substance or its phases, while potency is the absolute quantitative content of any drug substance or its phases. Potency and Purity Purity Purity is the qualitative content of […]
Potency and purity play a vital role in pharmaceutical analysis in managing quality, safety and efficacy of a pharmaceutical. Purity is the qualitative content of any drug substance or its phases, while potency is the absolute quantitative content of any drug substance or its phases.
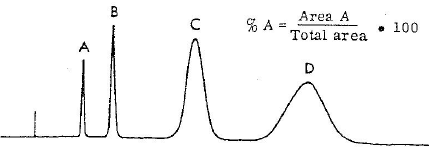
Purity is the qualitative content of any drug substance or its stages. It may be calculated by chemicals (titration), spectroscopic and chromatographic techniques (HPLC). Chemicals and spectroscopic methods of calculating the purity are not specific and selective and that is the reason these methods are rarely used for calculating the purity. But chromatographic methods are specific and selective and that is the reason chromatographic methods are used for calculating the purity in Pharmaceutical industries.
Purity is calculated by area normalisation method using the following formula:
Potency is the exact quantitative content of a drug substance or its stages. It is only applicable to standards. The highest pure material is used to calculate the potency. All types of impurities like organic impurities, inorganic impurities, residual solvents, counter ions, loss on drying or water content are taken into consideration for potency calculation.
First, purity is calculated as per the prescribed method of analysis. Then, after potency is calculated using the following formulae:
Potency = Purity – (Sulphated ash/residue of ignition + Loss on drying/water content/residual solvent + counter ion) etc..)
| Purity | Potency |
| It is qualitative content of a drug substance or a molecule | It is the quantitative value of the standard |
| It is the absolute value | It is an (absolute) exact value |
| Purity depends impurities present in the pharmaceuticals | Potency depends upon purity LOD/water, ROI or sulphated ash, in-organic impurities, counter ions, residual solvents etc. |
The following formula (given in the figure) is used to calculate potency from purity:
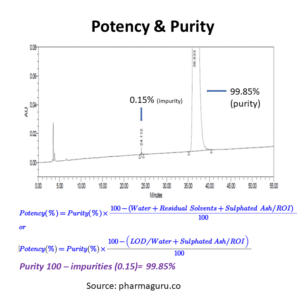
In summary, potency and purity are both integral to the pharmaceutical industry, but they refer to different characteristics of a drug. Potency focuses on the effectiveness of the drug in achieving its therapeutic purpose, while purity ensures that the drug is free from harmful impurities.
Potency and purity play a vital role in pharmaceutical analysis in managing quality, safety and efficacy of a pharmaceutical. Purity is the qualitative content of any drug substance or its phases, while potency is the absolute quantitative content of any drug substance or its phases.
Further Reading
Quick Links