Reverse Phase And Normal Phase HPLC are widely used in pharmaceutical development, still Reverse-Phase HPLC (RP-HPLC) is preferred over Normal-Phase HPLC (NP-HPLC) due to its better compatibility, broader analyte range, simpler method development, and easier system stabilisation. Reverse Phase And Normal Phase HPLC: Why Reverse Phase Is More Common The following 6 main key reasons explain […]
Reverse Phase And Normal Phase HPLC are widely used in pharmaceutical development, still Reverse-Phase HPLC (RP-HPLC) is preferred over Normal-Phase HPLC (NP-HPLC) due to its better compatibility, broader analyte range, simpler method development, and easier system stabilisation.
The following 6 main key reasons explain why Reverse-Phase HPLC is more commonly used than Normal-Phase HPLC:
1. Better Compatibility with Aqueous Samples
The biggest reason RP-HPLC is more commonly used is its compatibility with water-based (aqueous) mobile phases. Many compounds of interest in pharmaceuticals, biology, and environmental samples are polar and water-soluble. RP-HPLC allows for easy analysis of these compounds because:
2. Broader Range of Analytes
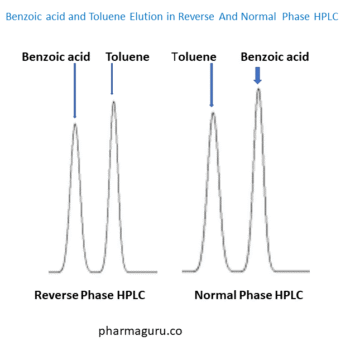
Reverse-phase columns, typically packed with non-polar stationary phases like C18 (octadecylsilane), retain a wide range of compounds—from small organics to complex biomolecules. RP-HPLC handles:
In contrast, NP-HPLC is better suited for non-polar and less polar compounds, which limits its range and usefulness.
3. More Stable and Reproducible Results
RP-HPLC tends to be more stable over time. That’s largely due to:
These factors lead to more reproducible and robust results in routine analyses.
4. Easier Method Development
Developing and optimising a reverse-phase method is generally easier due to:
This simplicity makes it the go-to method for labs that need reliable and quick turnaround on method development.
5. Cleaner, Less Hazardous Solvents
Normal-phase HPLC uses non-polar solvents like hexane, chloroform, or diethyl ether—solvents that are:
RP-HPLC, by contrast, uses water mixed with safer organic solvents like acetonitrile or methanol, making it the greener, safer choice for routine use.
6. More Common in Regulatory and Pharmaceutical Labs
Pharmaceutical regulatory guidelines (like those from the USP and ICH) often recommend reverse-phase methods for assay, content uniformity, and impurity profiling. Because of this:
While normal-phase HPLC still has its place-especially for separating very non-polar or structurally similar isomers, reverse-phase HPLC has become the workhorse of analytical labs around the world.
Its versatility, reproducibility, compatibility with water, and broader applicability across different industries make RP-HPLC the dominant choice for modern chromatographers.
Related
Related Video
Yes. While reverse-phase HPLC is more commonly used, normal-phase HPLC can be a better choice for separating non-polar compounds, chiral molecules, or structural isomers that reverse-phase HPLC cannot resolve efficiently. It’s also useful in lipid analysis and when dealing with compounds that are poorly soluble in water. However, method development and solvent handling are generally more complex in NP-HPLC.
In normal-phase HPLC, the stationary phase is polar (like bare silica), and the mobile phase is non-polar (like hexane). In reverse-phase HPLC, it’s the opposite: the stationary phase is non-polar (like C18-bonded silica), and the mobile phase is polar, typically a mix of water and an organic solvent like methanol or acetonitrile. This reversal in polarity is what gives reverse-phase HPLC its name.
Further Reading
Quick Links