
Explore a complete guide to Pharmaceutical Analysis in QC and ADL, covering QMS, calibration, documentation, troubleshooting, and key analytical techniques like HPLC, GC, MS, NMR, XRD, TLC, titration, and spectroscopy.
Pharmaceutical analysis is the qualitative and quantitative evaluation of pharmaceutical substances using validated analytical techniques to ensure quality, safety, and efficacy in compliance with GLP and GMP guidelines.
Pharmaceutical analysis is the qualitative and quantitative process of determining the identity, strength, purity, and composition of pharmaceutical substances and products using validated analytical techniques such as titrimetric, spectroscopic, chromatographic, mass spectrometric, and NMR methods. These analyses are performed to ensure product quality, safety, and efficacy, following approved analytical procedures such as STPs and pharmacopeial monographs, and are conducted under GLP and GMP–compliant environments.
Whether you are a graduate, postgraduate, or postdoctoral professional in Chemistry, Pharmacy, Quality Assurance/Quality Control (QA/QC), or Biotechnology, and have the passion to take on scientific and regulatory challenges, Pharmaceutical Analysis offers a highly rewarding career path.
The scope of Pharmaceutical Analysis is broad and multidisciplinary, encompassing both qualitative and quantitative evaluation of raw materials, intermediates, and finished pharmaceutical products to ensure their quality, purity, safety, and potency. These assessments are performed using advanced analytical techniques such as HPLC, GC, spectroscopy, mass spectrometry, and other modern instrumental methods, in compliance with global regulatory standards.
Beyond routine quality testing, Pharmaceutical Analysis plays a critical role across the entire drug lifecycle, including:
Pharmaceutical analysis is fundamental to ensuring the safety, quality, and efficacy of medicines throughout their entire lifecycle—from early drug development to post-market surveillance. It involves the identification of substances, accurate quantification of active ingredients, detection and control of impurities, and confirmation of correct dosage forms, ensuring that every medicine performs exactly as intended.
By validating that pharmaceutical products are pure, potent, stable, and consistent, pharmaceutical analysis plays a vital role in preventing adverse effects, therapeutic failures, and the circulation of counterfeit or substandard medicines. It safeguards public health, ensures patient safety, and maintains confidence in healthcare systems, while enabling manufacturers to meet stringent global regulatory requirements.
Pharmaceutical analysis is indispensable because it is directly linked to patient safety, therapeutic efficacy, and public trust. Any failure in analytical control can result in ineffective treatment, serious adverse reactions, regulatory non-compliance, or loss of confidence in healthcare systems.
By ensuring that medicines are safe, effective, and of uncompromised quality, pharmaceutical analysis acts as the scientific backbone of the pharmaceutical industry, protecting patients while upholding ethical standards and regulatory integrity across the global healthcare ecosystem
Pharmaceutical analysis plays a central role in ensuring the quality, safety, and efficacy of medicines throughout their lifecycle—from drug discovery and development to manufacturing and post-market surveillance. By applying validated analytical methods, it ensures that pharmaceutical products are pure, potent, stable, and perform as intended, while complying with stringent global regulatory standards.
It confirms the presence and identity of active pharmaceutical ingredients (APIs), verifies that excipients are safe and compatible, and ensures that the drug maintains its quality, safety, and therapeutic effectiveness throughout its shelf life, thereby safeguarding public health.
Pharmaceutical analysis ensures that medicines meet predefined quality specifications and remain consistent from batch to batch.
Pharmaceutical analysis minimises risks to patients by identifying and controlling potential hazards.
Pharmaceutical analysis ensures that medicines deliver the intended therapeutic effect consistently.
Pharmaceutical analysis is integrated across the entire drug lifecycle, ensuring continuous quality and safety assurance.
The following are the key pillars of Pharmaceutical Analysis:
The following are the key roles of QC in pharmaceutical analysis:
The Analytical Development Laboratory (ADL) plays a vital role in analytical method development and validation in the pharmaceutical industry, ensuring the identity, purity, strength, and stability of drug substances and drug products. Operating under stringent global regulatory guidelines such as ICH and FDA, ADL ensures that analytical methods consistently generate accurate, precise, and reliable data, thereby supporting drug quality, safety, and patient consistency.
ADL serves as a critical link between Research & Development (R&D) and Quality Control (QC) by developing robust, reproducible, and lifecycle-oriented analytical methods. These methods are designed to withstand routine QC usage while supporting regulatory submissions and commercial manufacturing. The following are the key responsibilities of ADL in Pharmaceutical Analysis:
The Analytical Development Laboratory (ADL) and Quality Control (QC) serve distinct but complementary roles in pharmaceutical analysis. ADL is responsible for developing, optimising, validating, and transferring analytical methods, while QC is responsible for executing these validated methods for routine testing of pharmaceutical raw materials, in-process samples, and finished products.
In essence, ADL designs and establishes analytical methods, ensuring their robustness and regulatory compliance, whereas QC applies these methods consistently to ensure batch-to-batch quality, safety, and efficacy during commercial manufacturing.
The following are the different pillars of QMS:
GLP is an international quality system standard for planning, performing, monitoring, recording, reporting, and archiving non-clinical health and environmental safety studies, ensuring data is reliable, consistent, and valid. It is mainly applicable to R&D, like analytical research and chemical research.
ALCOA data integrity represents the core principles: Attributable, Legible, Contemporaneous, Original, and Accurate, required by regulatory bodies such as the FDA to ensure trustworthy and reliable data throughout its lifecycle. Its extension, ALCOA+ (Complete, Consistent, Enduring, Available), provides a comprehensive framework for maintaining data quality, security, and compliance in regulated industries, particularly pharmaceuticals.
Change Control, Deviation, CAPA (Corrective and Preventive Action), and OOS (Out of Specification) are interconnected quality management processes in pharmaceuticals. Change Control manages planned changes, Deviations and OOS handle unexpected events or non-conformances, and CAPA implements corrective and preventive measures. Together, these processes ensure product quality, regulatory compliance, and continuous improvement.
Both calibration and qualification are essential for ensuring quality control in pharmaceutical analysis. Instrument calibration verifies and adjusts an instrument’s accuracy against known standards to ensure precise and reliable measurements. Instrument qualification—covering Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ)—confirms that equipment is properly installed, operates correctly, and consistently delivers expected results. Together, calibration and qualification form a continuum: calibration ensures accurate readings, while qualification validates that the system is fit for its intended use.
The following are the different pillars of Instrument Calibration and Qualification:
The following analytical techniques are widely used in pharmaceutical analysis
The following are the main pillars of Classical (Wet Chemistry) Methods:

Read the complete article: Titration
Gravimetric analysis is a quantitative analytical technique in which the amount of an analyte is determined by measuring the mass of a chemically related, pure compound. The analyte is typically converted into a stable solid precipitate that is filtered, washed, dried, and accurately weighed, and its original quantity is calculated using stoichiometric relationships. Common pharmaceutical examples include the sulphated ash test and gravimetric titration.
Read the complete article: Limit tests
Read the complete article: HPLC Method Development
Read the complete article: Analytical Method Validation
Read the complete article: AMT
Read the complete article: SOP & STP
Specifications define the acceptable quality limits for pharmaceutical products.
They may include limits for:
Specifications are approved by regulatory authorities and must be strictly followed.
Good Documentation Practices (GDP/GDocP) are guidelines that ensure records are accurate, legible, complete, and traceable in regulated industries such as pharmaceuticals and medical devices. Based on ALCOA principles (Attributable, Legible, Original, Contemporaneous, Accurate, and Complete), GDP governs the creation, review, storage, and retention of documents to maintain data integrity, ensure regulatory compliance, and support product quality. Key practices include timely recording, use of indelible ink, avoidance of ditto marks, maintenance of clear audit trails, and controlled document handling, thereby building trust in data and processes.
Read the complete article on HPLC Troubleshooting
Read the complete article on GC troubleshooting
Spectroscopy and Instrument-Related Issues involve common challenges such as wavelength inaccuracies, baseline drift, noise, lamp or detector failure, and improper calibration that can affect analytical accuracy and reproducibility. Effective troubleshooting, routine calibration, preventive maintenance, and adherence to SOPs are essential to ensure reliable spectroscopic data and regulatory compliance in pharmaceutical analysis.
Analytical errors may arise from:
Validation, calibration, training, and SOPs help minimise errors.
Read the complete article on Human Errors in Pharmaceutical Analysis
Read the complete article on Audit & Inspections
Audit Readiness for QC and ADL Laboratories refers to the continuous state of compliance with GMP, GLP, and regulatory guidelines through proper documentation, data integrity (ALCOA+), validated methods, calibrated instruments, and trained personnel. Maintaining audit-ready labs ensures smooth regulatory inspections, minimises observations, and demonstrates consistent control over analytical processes in pharmaceutical quality systems.
Common regulatory observations, particularly from agencies such as the U.S. Food and Drug Administration (FDA), frequently relate to weaknesses in quality systems, documentation, training, and data integrity. These deficiencies are often cited in Form FDA 483 observations and may indicate potential non-compliance with the FD&C Act, GMP regulations, and ICH guidelines, posing risks to product quality, patient safety, and regulatory approval.
To prevent common regulatory observations, pharmaceutical QC and ADL laboratories should maintain a proactive, compliance-driven quality culture supported by robust systems and continuous oversight.
By embedding these practices into daily operations, organisations can minimise regulatory risk, ensure compliance, and consistently deliver high-quality pharmaceutical products.
Understanding pharmaceutical analysis terminology is essential for students, analysts, researchers, and professionals in R&D, Quality Control (QC), Quality Assurance (QA), and Regulatory Affairs to ensure effective communication, regulatory compliance, and accurate interpretation of analytical data. The following terminology is widely used in pharmaceutical analysis:

Pharmaceuticals include Active Pharmaceutical Ingredients (APIs), excipients, and finished dosage forms intended for diagnosis, treatment, or prevention of diseases.
Dosage forms are the final products administered to patients, such as:
Each dosage form has unique physical and chemical characteristics that determine the analytical approach, sample preparation, and testing methods.
A method of analysis is a scientifically validated approach used to test pharmaceutical materials. It defines how an analyte is identified, quantified, or characterised.
A method of analysis ensures:
An analytical procedure is a step-by-step description of how an analysis is performed. It includes:
System Suitability Testing verifies that the analytical system is performing correctly before or during sample analysis. SST is mandatory for chromatographic methods.
Read the complete article: How to decide SST?
SST parameters may include:
Qualitative tests identify the presence or nature of substances.
Examples:
Quantitative tests measure the amount of analyte present.
Examples:
Read the complete article: Qualitative & Quantitative Tests
The following common calculation methods are widely used in pharmaceutical analysis:
Read the complete article: Calculation in the chromatographic method
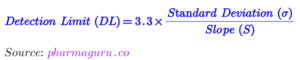
Sensitivity refers to the ability of a method to detect small changes in analyte concentration.
Read complete article: DL & QL
Pharmaceutical standards serve as references for comparison and calibration.
Highly pure substances with certified values used for method validation and calibration.
Official reference standards supplied by pharmacopeial bodies.
Qualified standards derived from primary standards for routine laboratory use.
Read the complete article: Reference Vs Working standard
Calibration ensures that analytical instruments produce accurate and reliable results.All Instruments used in QC/ADL must be calibrated as per the respective SOP, such as:
Key regulatory and scientific guidelines include:
These guidelines ensure global compliance and harmonisation.
Career opportunities in Quality Control (QC) and Analytical Development Laboratory (ADL) are rapidly growing across the pharmaceutical, biotechnology, and manufacturing industries. These roles focus on analytical testing, method development, regulatory compliance, validation, and continuous process improvement, offering strong long-term career growth for trained professionals.
Automation and digitalisation in pharmaceutical analysis enhance accuracy, efficiency, data integrity, and regulatory compliance by integrating advanced analytical instruments, laboratory automation, and digital systems such as LIMS, ELN, CDS, and data integrity tools. These technologies reduce manual errors, improve throughput, enable real-time monitoring, and support ALCOA+ compliance, making analytical laboratories more reliable, audit-ready, and aligned with modern regulatory and Industry 4.0 expectations.
The future of analytical technologies in pharmaceutical analysis is driven by advanced automation, artificial intelligence (AI), machine learning, and real-time analytics, enabling faster, more accurate, and predictive decision-making. Innovations such as Process Analytical Technology (PAT), continuous manufacturing analytics, miniaturised instruments, and digital twins will enhance quality by design (QbD), strengthen data integrity, and improve efficiency across the drug lifecycle, supporting smarter, compliant, and patient-centric pharmaceutical development.
Many Active Pharmaceutical Ingredients (APIs) are chiral in nature, meaning they exist as non-superimposable mirror images known as enantiomers. Since different enantiomers can show significantly different pharmacological, toxicological, and metabolic profiles, regulatory authorities require chiral purity testing and Specific Optical Rotation (SOR) analysis to ensure drug safety and efficacy.
This article highlights commonly used chiral APIs that mandate optical rotation and enantiomeric purity evaluation as part of routine pharmaceutical quality control.
Paracetamol, also known as Acetaminophen, is one of the most widely used analgesics and antipyretic drugs globally. Due to its extensive therapeutic use, stringent pharmaceutical analysis and quality control are essential to ensure safety, efficacy, and regulatory compliance. This case study outlines the analytical evaluation of Paracetamol API as per pharmacopeial and ICH guidelines.
Acetaminophen API contains Not Less Than (NLT) 98.0% and Not More Than (NMT) 102.0% of C₈H₉NO₂, calculated on a dried basis.
Paracetamol appears as a white or almost white crystalline powder, odorless, with a slightly bitter taste. It is freely soluble in alcohol and sparingly soluble in water.
The Fourier Transform Infrared (FTIR) spectroscopy spectrum of the sample matches the reference standard, confirming the presence of characteristic functional groups such as:
Using High Performance Liquid Chromatography (HPLC), the retention time of the sample peak corresponds with that of the Paracetamol reference standard, confirming identity.
The assay of Paracetamol is performed using HPLC.
| Impurity | Specification |
|---|---|
| Acetaminophen Related Compound B | NMT 0.05% |
| Acetaminophen Related Compound C | NMT 0.05% |
| Acetaminophen Related Compound D | NMT 0.05% |
| Acetaminophen Related Compound J | NMT 0.001% |
| Individual Unspecified Impurity | NMT 0.05% |
| Total Impurities | NMT 0.10% |
Result: All specified and unspecified impurities are within acceptable ICH and pharmacopeial limits.
This pharmaceutical analysis case study demonstrates that Paracetamol (Acetaminophen API) fully complies with pharmacopeial standards, ICH guidelines, and regulatory requirements. Comprehensive testing—including identity, assay, impurities, heavy metals, and residual solvents—ensures high product quality, patient safety, and regulatory acceptance.
Chiral purity and Specific Optical Rotation (SOR) testing are essential quality attributes for many modern APIs. Drugs such as Ezetimibe, Ibuprofen, Pregabalin, Rosuvastatin Sodium, and Atorvastatin Calcium require strict stereochemical control to ensure therapeutic efficacy, patient safety, and regulatory compliance.
Incorporating validated chiral analytical methods strengthens pharmaceutical quality systems and supports global regulatory acceptance.
Understanding the terminology related to pharmaceutical analysis, including carriers, analytical methods, standards, techniques, tests, and guidelines, is fundamental for anyone working in the pharmaceutical industry. These concepts form the backbone of quality assurance, regulatory compliance, and scientific decision-making. A strong foundation in pharmaceutical analysis ensures the development and delivery of safe, effective, and high-quality medicines.
Pharmaceutical analysis is the branch of pharmaceutical science concerned with the qualitative and quantitative evaluation of drugs, excipients, and dosage forms to ensure their identity, strength, purity, quality, safety, and efficacy using validated analytical methods under GLP and GMP conditions.
The scope of pharmaceutical analysis includes:
1. Drug development and formulation studies
2. Quality control and quality assurance
3. Regulatory compliance and documentation
4. Stability studies
5. Method development and validation
6. Impurity profiling and assay determination
The methods of pharmaceutical analysis include:
1. Chemical methods (titrimetry, gravimetry)
2. Instrumental methods (UV, IR, HPLC, GC, LC-MS)
3. Biological methods
4. Microbiological methods
The two main types of pharmaceutical analysis are:
1. Qualitative analysis – identification of drugs and impurities
2. Quantitative analysis – determination of the amount or concentration of drugs
The four types of chemical analysis are:
1. Qualitative analysis
2. Quantitative analysis
3. Volumetric (titrimetric) analysis
4. Gravimetric analysis
Pharmaceutical analysis offers career opportunities in:
1. Quality Control (QC) laboratories
2. Research and Development (R&D)
3. Analytical method development and validation
4. Regulatory affairs support
5. Stability and compliance studies
6. Pharmaceutical and biotechnology industries
Examples of pharmaceutical analysis include:
1. Assay of tablets by HPLC
2. Impurity profiling using LC-MS
3. Dissolution testing of oral dosage forms
4. Identification of drugs by IR spectroscopy
5. Water estimation by Karl Fischer titration
Neither is universally better; the choice depends on career goals.
Pharmaceutics is better for formulation development, drug delivery systems, and product design.
Pharmaceutical analysis is better for quality control, regulatory compliance, and analytical research.
Choose pharmaceutical analysis if you prefer instrumentation, data interpretation, and laboratory-based work.
Further Reading:
Quick Links