Understand potency, purity, and assay in pharma testing. Discover key differences, how to calculate them, and why they matter in drug development
Table of Content
Potency, Purity, and Assay: Core Concepts in Pharmaceutical Analysis
Potency defines the exact quantitative or biological activity of a drug substance; purity characterises its chromatographic profile and level of impurities; and assay provides a validated quantitative determination of the active pharmaceutical ingredient relative to an established reference standard.
Potency, purity, and assay are fundamental yet often misunderstood terms in pharmaceutical analysis. They play a critical role in drug development and quality control. Whether it’s qualitative analysis, such as pharmaceutical identification using techniques like HPLC, GC, UV, FTIR, GC-MS, or LC-MS, or quantitative analysis, including assays and content testing, these parameters are always at the forefront, with standards guiding every step.
Despite their widespread use, many chemists face confusion when it comes to correctly understanding and applying these concepts. That’s why I’ve decided to share my practical, experience-based insights into potency, purity, and assay.
In this article, I’ll discuss:
Whether you’re a student, analyst, or industry professional, this guide will help you gain clarity and confidence in handling these crucial pharmaceutical parameters.

Related: Relative Response Factor (RRF) in Pharmaceutical Analysis: Learn In 5 Steps
Potency is the exact quantitative content of a pharmaceutical. It is only applicable to standards and used for assay, purity and impurity profile calculation. It refers to the strength of the pharmaceutical. It is the absolute value.
The highest pure material is used to calculate the potency. All types of impurities, like organic impurities, inorganic impurities, residual solvents, counter ions, loss on drying or water content, are taken into consideration for potency calculation.
Purity is the qualitative content of any pharmaceutical. It represents how free a pharmaceutical is from impurities.
Purity typically refers to chromatographic purity and is commonly expressed as peak area percentage (area%). It represents the proportion of the main pharmaceutical/analyte peak area relative to the total area of all detected peaks in a chromatogram, as determined by high-performance liquid chromatography (HPLC) or gas chromatography (GC)
It can also be calculated by other analytical techniques, like chemical and spectroscopic techniques. Chemicals and spectroscopic methods of calculating the purity are not specific and selective, and that is the reason these methods are rarely used for calculating the purity. But chromatographic methods are specific and selective, and that is the reason chromatographic methods are widely used for calculating the purity in the Pharmaceutical industries.
The Assay is the quantitative content of a pharmaceutical. It is a relative value and it is calculated against its corresponding standard while using the chromatographic technique or spectroscopic technique. The potency of the standard is required in the assay calculation while using the chromatographic technique or spectroscopic technique.
The Assay can also be calculated using the chemical titration technique and in this technique, there is no need of any external standard. Since the chemical technique is not specific and that is why it is rarely used in the pharmaceutical industries.
Related Video:
You may like: Relative Response Factor (RRF) in Pharmaceutical Analysis
The highest pure material is used to calculate the potency. All types of impurities, like organic impurities, inorganic impurities, residual solvents, counter ions, loss on drying or water content, are taken into consideration for potency calculation.
It is typically determined using a mass balance approach and is reported as the percentage ratio of the weight of the main pharmaceutical/analyte to the total weight of the drug substance (wt/wt %). This calculation accounts for all impurities present in the drug substance, including residual solvents, water content, related substances, and inorganic impurities, providing a true measure of the actual content and quality of the pharmaceutical.
Potency is calculated from purity in chromatography methods like HPLC and GC using the following formula-1 and formula -2:
Formula-1 and formula -2:
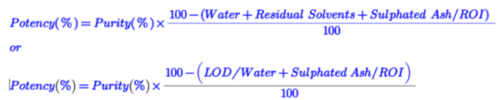
Note:
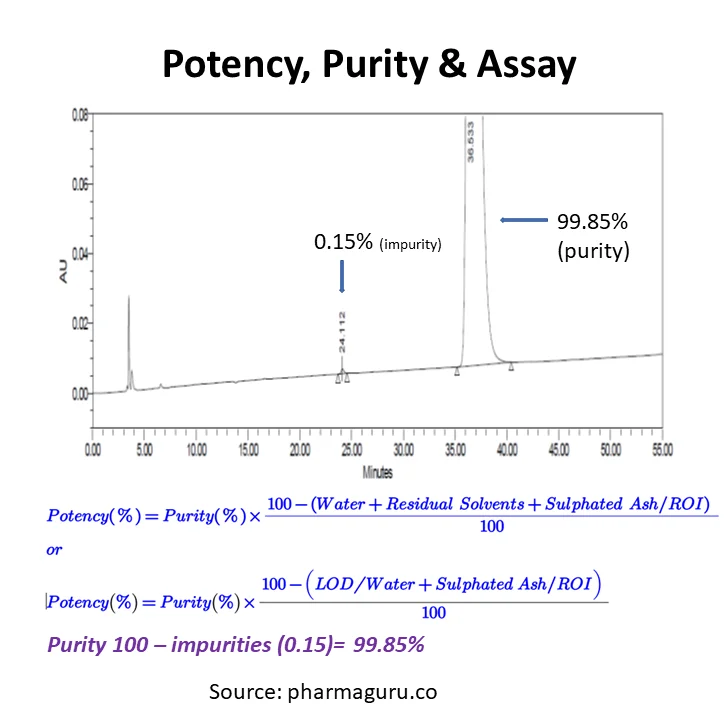
If a drug substance has an impurity A is 0.15%, LOD is 1.0%, and Sulphated ash is 0.1%. Calculate the potency
Using Formula-1 (purity: 99.85%, Sulphated ash 0.10% and LOD 1.0%)
For drug substances that exist in salt form, potency can be reported in two ways:
The choice between these depends on regulatory requirements and nature of the pharmaceutical.
The following analytical techniques are widely used in the pharmaceutical industries in for potency calculations:
Note: The Titrimetric Method is not specific and selective, and that is the reason it is rarely used for potency calculation
Purity can be calculated using chromatographic techniques like high-pressure liquid chromatography , Gas chromatograph etc as per method requirements. The following methods are used in the industries for calculating the purity:
Calculate the percentage purity by the following formula (Formula-3) by area normalisation method or area% method:
Formula-3:

Note: Impurity peaks having a value ≥QL( Quantitation limit) are considered in purity calculation
In this method, first each impurity is calculated against either a diluted standard (see external standard assay calculation formula-4 in assay section) of the main analyte or against their corresponding impurity (as per method) and then purity is calculated using the following formula:
Formula-4
Note*:
If a drug substance has an impurity A is 0.15%. calculate purity
Using Formula-3
Using Formula-2
If the area response of impurity A of a drug substance is 7500 mAU and the area response of the main analyte is 50000 mAU; then calculate the purity
% Purity = Area response of main analyte/Sum of area of Impurity I and Main Analyte = 500000/50750 = 99.85%
Assay by chromatographic techniques (HPLC and GC) is a widely used for quantifying the amount of main pharmaceutical/Analyte in a pharmaceutical. The measurement is made by comparing the main analyte/pharmaceutical peak area response in the sample to that of a reference standard with a known potency. The assay result is calculated using the sample response factor and standard response factor and potency.
Assay results can be reported in two ways:
The External standard method and Internal standard method are used for assay calculation by chromatographic methods like HPLC and GC
Formula-4:

| Purity | Potency | Assay |
| Potency depends upon purity LOD/water, ROI or sulphated ash, inorganic impurities, counter ions, residual solvents, etc. | It is the quantitative value of a drug substance or a molecule | It is the absolute value |
| It is the absolute value | It is a relative value | Potency depends upon purity LOD/water, ROI or sulphated ash, inorganic impurities, counter ions, residual solvents etc. |
| Purity depends upon related organic impurities | It is the quantitative value of the standard | Assay depends upon potency ( while using spectroscopic and chromatographic techniques). |
Note:
The following factors affect the potency:
Pharmaceutical analysis like Assay, Related substances by external method, Content test and Residual solvent tests depend on the use of a reference standard with accurately assigned potency, typically determined through a mass balance approach. The reliability of these tests are closely tied to the precision and accuracy of the analytical method employed. If the potency of the reference standard is inaccurate, this error will directly impact the accuracy of the assay results. Additionally, using different reference standards or employing varying methods to determine the purity of the reference standard can lead to inconsistent assay results, even when analyzing the same drug substance.
Potency plays a vital role in:
Assay on as is basis of the highest pure material can be considered as a potency
Regulatory guidelines for potency, purity, and assay are critical in pharmaceutical development and manufacturing. These parameters ensure that a drug product is safe, effective, and of consistent quality.
| Components (Potency/Purity/Assay) | Key Guidelines | Highlights |
| Potency | FDA 21 CFR 211, ICH Q6A, USP <1033> | Measures of pharmaceutical content and potency is required to calculated the assay |
| Purity | ICH Q3A/B/C, USP <1086>, 21 CFR 211 | Purity depends upon impurities and covers organic/inorganic impurities, residual solvents |
| Assay | CH Q2(R2), USP, FDA Guidance | Measures of pharmaceutical content and potency is required to calculate the assay |
The following units are widely used for potency:
1. %W/W (Weight/Weight
2.0 Milligrams (mg), Micrograms (mcg/µg), or Grams (g)
3.0 International Units (IU)
4.0 Units (U)
5.0 Activity Units (AU)
6.0 Equivalents (Eq, mEq, µEq)
Note:
Purity, potency, and assay are closely interconnected and fundamental concepts in the pharmaceutical industry. They play a crucial role in ensuring the safety, efficacy, and quality of drug products. Understanding these parameters is essential for compliance with regulatory standards and for maintaining product integrity throughout its life cycle.
If you have any questions or need further clarification on any of these topics, feel free to leave a comment below. I’ll be happy to respond and assist you as a priority!
Related
Potency is calculated by the following formulae:
Potency = Purity – (Sulphated ash/residue of ignition + Loss on drying/water content/residual solvent + counter ion) etc..)
No. Purity is the qualitative value, whereas the assay is the quantitative value. Secondly, for chromatographic analysis, purity is the absolute value, whereas assay is the relative value.
Potency is not calculated from assay, but the assay is calculated from potency. Potency is calculated from purity.
Both purity and potency tell about the exactness of the pharmaceutical. Purity is the qualitative value whereas potency is the quantitative value. Secondly, potency is only applicable for standard whereas purity is applicable for both standard and sample.
Purity, potency and assay tell about the exactness of the pharmaceutical. Purity is the qualitative value whereas potency and assay are the quantitative value. Secondly, potency is only applicable to standard whereas purity is applicable to both standard and sample. Assay is applicable to sample.
Assay (%w/w) = (Test area ÷ Standard area) × (Standard weight. ÷Test weight) × (Test Volume ÷ volume) x Potency
Percentage purity is calculated by normalisation method and external standard method. The following formulae is used to calculate the purity:
Purity= (100 – sum of all impurities* present in the chromatogram)
Potency is the exact quantitative content of a drug substance or its stages and It is only applicable to standard.
Potency = Purity – (Sulphated ash/residue of ignition + Loss on drying/water content/residual solvent + counter ion) etc..) and hence Purity = Potency + (Sulphated ash/residue of ignition + Loss on drying/water content/residual solvent + counter ion) etc..)
HPLC purity is the qualitative value, whereas the HPLC assay is the quantitative value. Secondly, HPLC purity is the absolute value, whereas HPLC assay is the relative value.
Assay (%w/w) = (Test area ÷ Standard area) × (Standard weight. ÷Test weight) × (Test Volume ÷ volume) x Potency
Potency = Purity – (Sulphated ash/residue of ignition + Loss on drying/water content/residual solvent + counter ion) etc..)
Assay (%w/w) = (Test area ÷ Standard area) × (Standard weight. ÷Test weight) × (Test Volume ÷ volume) x Potency
Yes. A sample might be pure but contain less than the expected API amount.
Not exactly. Potency is more related to strength (often from formulation), while assay is a lab measurement of API content.
Impurities may be toxic or reduce the drug’s shelf life, even if the API is effective.
Abbreviations
Further Reading:
Quick Links