Understand the importance of Detection Limit (DL) and Quantification Limit (QL) in analytical method validation. Learn definitions, regulatory guidelines, and how to determine DL and QL in pharmaceutical analysis.
Detection Limit (DL) and Quantification Limit (QL) in Analytical Method Validation
Detection Limit (DL) and Quantification Limit (QL) are critical parameters in analytical method validation, especially in pharmaceutical analysis where accurate reporting of trace-level analytes is essential.
The Detection Limit (DL) is defined as the lowest concentration of an analyte that can be detected, but not necessarily quantified, with a certain degree of confidence. It represents the point at which the analyte signal can be reliably distinguished from background noise. DL is typically used in qualitative determinations – such as impurity profiling or limit tests – but may also be applicable in some quantitative analyses. The ICH Q2(R1) guidelines provide specific recommendations for determining DL based on whether the method is instrumental or non-instrumental.
The Quantification Limit (QL) is the lowest concentration of an analyte that can be quantitatively determined with acceptable precision and accuracy. It reflects the method’s ability to produce reliable results at low concentration levels. QL is essential for the quantitative determination of impurities, degradation products, and trace-level components in pharmaceutical formulations.
This article explains the concepts, determination procedures, and regulatory expectations for DL and QL, helping you understand their roles in ensuring analytical method reliability.
You may like:
The following three methods are available to calculate Detection Limit (DL).
Out of above methods, Signal to noise ratio (S/N) method is widely used in the industries. Prepare several analyte solutions at lower concentrations. Inject each solution one by one and check the S/N ration. The lowest concentration which gives S/N ratio about 2:1 to 3:1 will be DL concentration.
A drug substance D having the following specifications for related substances:
Sample concentration in the method is 1.0 mg/ml to perform related substances test.
Prepare Impurity A at (lower concentration) 0.01% that is 1000 x 0.01/100 = 0.1mcg/ml. Inject this solution in triplicate and note down S/N ration in each injection.
| Injection | S/N ratio | Area response |
| 1 | 3 | 285 |
| 2 | 4 | 210 |
| 3 | 3 | 350 |
Conclusion
Note: Similarly DL can also be calculated for unknown impurities by preparing main analyte concentration at lower level
The following three methods are available to calculate Quantitation Limit (QL).
Out of above methods, Signal to noise ratio (S/N) method is widely used in the industries.. Prepare several analyte solutions at lower concentrations. Inject each solution one by one and check the S/N ration. The lowest concentration which gives S/N ratio about 10 is the QL concentration.
A drug substance D having the following specifications for related substances:
Sample concentration in the method is 1.0 mg/ml to perform related substances test.
QL of Impurity A
1.0 mg/ml sample concentration is equivalent 1000 mcg/ml
Prepare Impurity A at (lower concentration) 0.03% that is 1000 x 0.03/100 = 0.3mcg/ml. Inject this solution in six times. Calculate the RSD of six injections and note down S/N ratio of each injection.
| Injection | S/N ratio | Area response |
| 1 | 11 | 1100 |
| 2 | 12 | 1123 |
| 3 | 10 | 1110 |
| 4 | 13 | 1125 |
| 5 | 12 | 1140 |
| 6 | 12 | 1115 |
| RSD | NA |
Conclusion:
Detection Limit (DL) is also called Limit of Detection(LOD) and Quantification Limit (QL) is called Limit of Quantification (LOQ)
Note: DL and QL definition will also be applicable for LOD and LOQ
The following formula are used for calculation of DL and QL
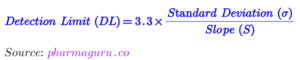
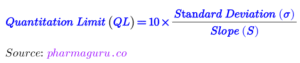
The detection limit (LOD) and quantification limit (LOQ) are both terms used in pharmaceutical analysis to describe the sensitivity of a measurement method, but they refer to different thresholds:
1.Detection Limit (DL):
2. Quantification Limit (QL):
Summary Table
| Component | Detection Limit (DL) | Quantification Limit (QL) |
| What it indicates | Presence | Amount |
| Confidence level | Low | High |
| Typical multiplier | 3× standard deviation | 10× standard deviation |
| Precision | Not required | Required |
DL and QLare the important parameters of the Analytical method validation. Both knowledge and experience are required to perform the same. Now I hope this article, cleared all your doubts and now you can independently perform DL and QL during method development and method validation. For any opinion or suggestions related to this article, you can write in the comment section. For any further assistance you can contact me using contact form.
Related:
Disclaimer: The numerical data used in the tables or calculations are not actual data. It is designed to explain the topic.
Quick Links