pH Meter: The Backbone of Drug Development
pH meter is the backbone of drug development, because without accurate pH measurement, the quality and safety of neither raw materials, intermediates, nor active pharmaceutical ingredients, nor pharmaceutical formulations can be guaranteed. pH Meter: The Backbone of Drug Development 1. How pH Meter is the Backbone of Drug Development The pH meter plays a vital […]
Reference Standard (RS) Vs Working Standard (WS) In Drug Development: Learn With FAQs
A Reference Standard (RS) is a highly purified, officially recognised substance that serves as the “gold standard” for identity, strength, quality, and purity in pharmaceutical analysis. In contrast, a Working Standard (WS) is a secondary standard, derived from and rigorously calibrated against the RS. While both are essential to maintaining the accuracy and reliability of […]
Degradation Products Vs Impurities: Key Differences With 7+FAQs
Degradation products are a type of impurity formed when a substance chemically breaks down over time due to factors like heat, light, or moisture. While all degradation products are impurities, not all impurities are degradation products—some arise from manufacturing, excipients, or environmental contamination. Degradation Vs Impurities: FAQs You May Like: Degradation Vs Impurities: Key Differences […]
Tautomerism Vs Rotamerism In Drug Development: Interview Questions
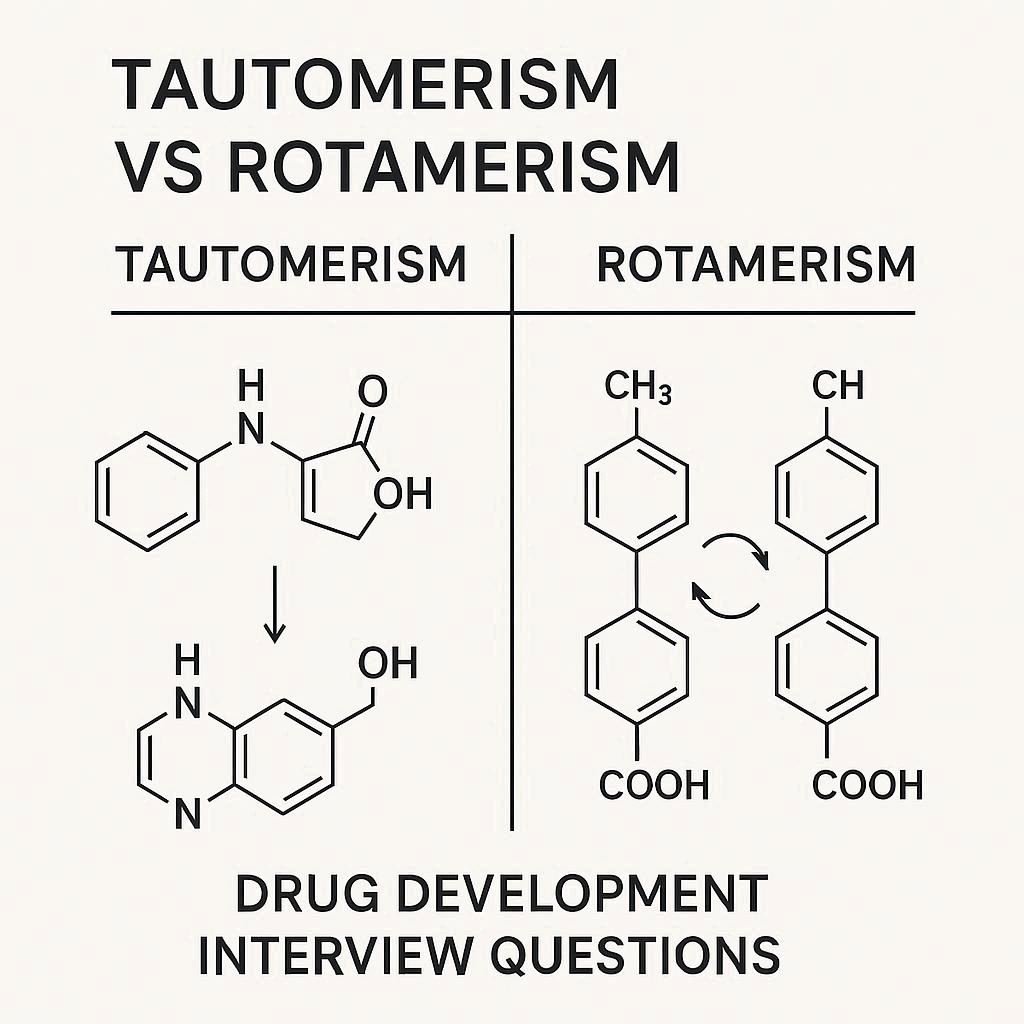
Tautomerism in pharmaceuticals refers to the interconversion of structural isomers via the migration of a hydrogen atom, while rotamerism involves the interconversion of conformational isomers through rotation around a single bond. Both processes can profoundly influence a drug’s stability, bioavailability, and pharmacological activity, with tautomeric transitions typically requiring a higher energy barrier than rotameric changes. […]
Pharmaceutical Regulatory Agencies and Their Key Functions: Get Mastery With 9+ FAQs

Major Pharmaceutical Regulatory Agencies include the U.S. Food and Drug Administration (FDA), the European Medicines Agency (EMA), Japan’s Pharmaceuticals and Medical Devices Agency (PMDA), India’s Central Drugs Standard Control Organisation (CDSCO), and the UK’s Medicines and Healthcare products Regulatory Agency (MHRA). These national and international bodies are responsible for ensuring the safety, efficacy, and quality […]
Sample Preparation For HPLC Analysis: Interview Questions

Sample preparation for HPLC analysis involves converting the sample into a clear, soluble solution at an appropriate concentration for injection. Techniques such as sonication, stirring, dissolution, filtration, and solvent extraction are employed to dissolve the sample, remove impurities, simplify complex matrices, and protect the HPLC column. The chosen method depends on the sample matrix, analyte […]
Top Interview Questions On CTD and DMF in the Pharmaceutical Industry

CTD and DMF are related but distinct in drug regulation. The CTD is the standardised format for submitting regulatory applications, while a DMF is a specific submission that provides confidential details about a drug’s manufacturing process. In essence, the CTD defines the structure of the application, and the DMF contains the confidential information about an […]
Change Control Vs Deviation in Pharma: Get Mastery With FAQs
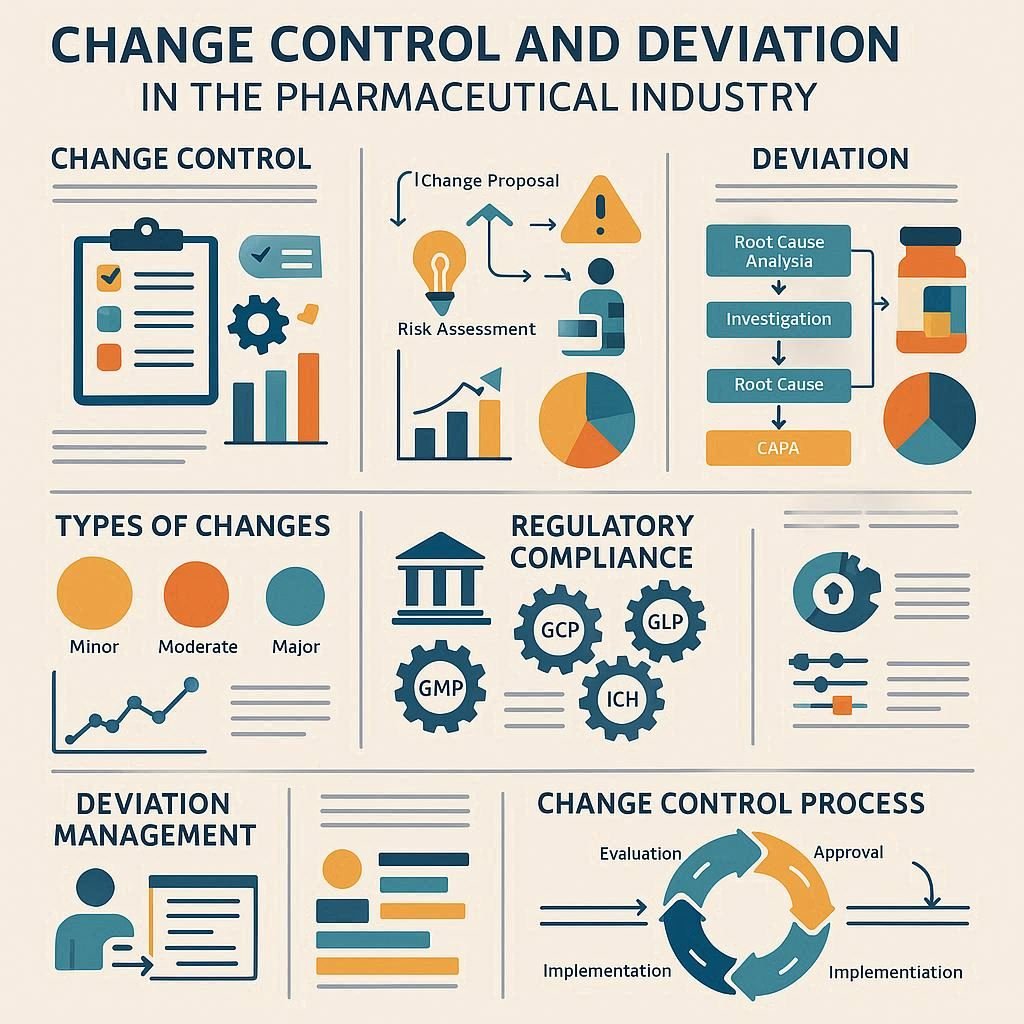
Change control manages planned modifications to processes or systems through a formal approval process, ensuring quality and risk mitigation. In contrast, a deviation is an unplanned, unexpected departure from established procedures that requires investigation and often leads to Corrective and Preventive Actions (CAPA) to address the issue and prevent recurrence. Change Control Vs Deviation in […]
Pharmaceutical Specifications Vs Pharmaceutical Tests: Interview Questions

Pharmaceutical specifications define the quality standards a drug product must meet, including acceptance criteria and referenced test methods. Pharmaceutical tests are the laboratory procedures used to measure product characteristics and verify compliance with these specifications. In short, specifications describe what quality is required, while tests define how that quality is measured. Pharmaceutical Specifications vs Pharmaceutical […]
Photostability and Forced Degradation Studies Of Pharmaceuticals: Interview Questions
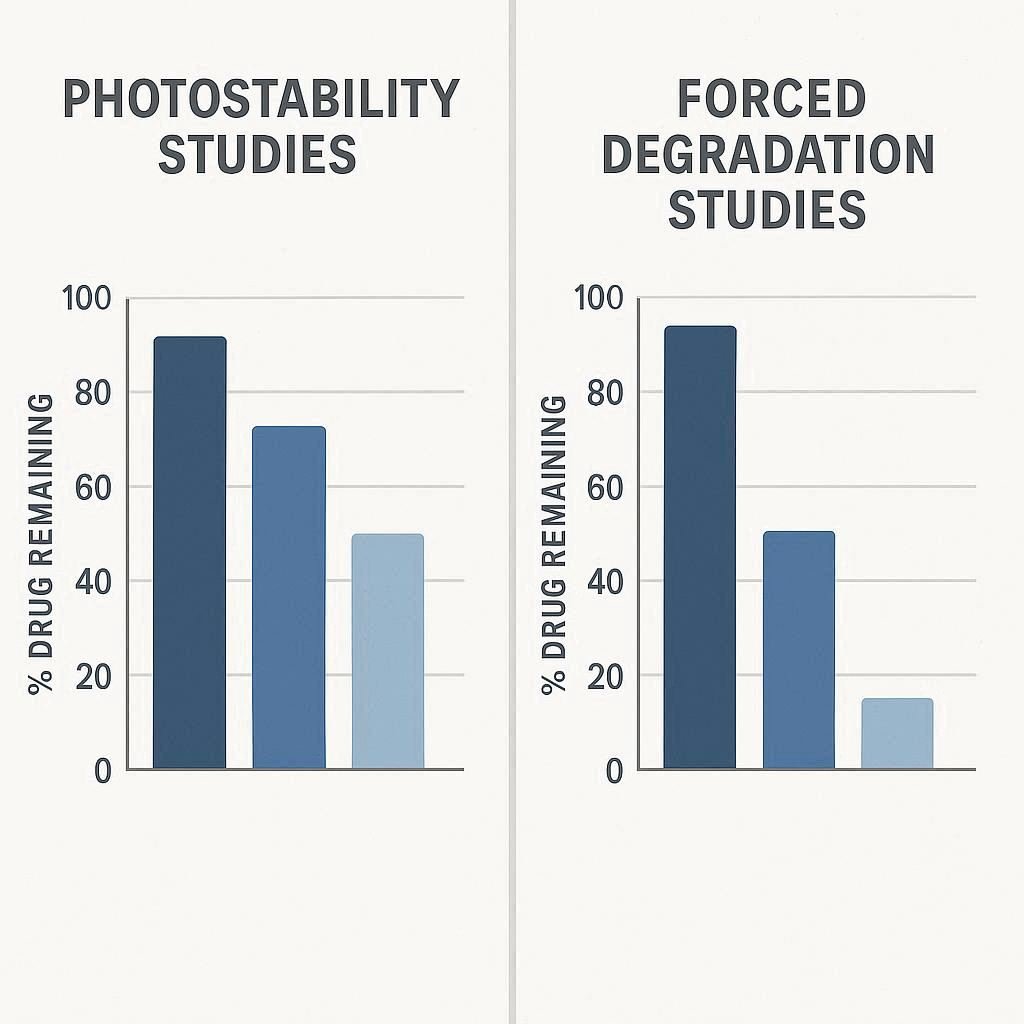
Photostability and forced degradation studies are stress-testing methods used in drug development to understand degradation pathways. Forced degradation uses extreme conditions (e.g., heat, pH, oxidation, light) to identify all potential degradation products and support stability-indicating method development. Photostability, a specific subset, focuses solely on light exposure to assess photosensitivity and determine the need for light-protective […]
