Partition, adsorption, ion exchange, and size exclusion chromatography are all powerful separation techniques and are widely used in pharmaceutical development. Partition chromatography separates components based on their relative solubility between two immiscible liquid phases. Adsorption chromatography relies on differences in how strongly compounds bind to a solid stationary phase. Ion exchange chromatography separates molecules according […]
Partition, adsorption, ion exchange, and size exclusion chromatography are all powerful separation techniques and are widely used in pharmaceutical development.
Partition chromatography separates components based on their relative solubility between two immiscible liquid phases. Adsorption chromatography relies on differences in how strongly compounds bind to a solid stationary phase. Ion exchange chromatography separates molecules according to their net charge and their interaction with charged resins. Size exclusion chromatography, on the other hand, separates solely based on molecular size, with larger molecules eluting first as they are unable to enter the pores of the stationary phase.
Adsorption is a separation technique based on the ability of certain molecules to adhere to the surface of a solid material (the adsorbent). Because different molecules interact with the adsorbent to different extents, selective adsorption occurs, allowing specific components to be captured while others pass through
Application
In the food industry, adsorption is commonly used for clarifying liquids, removing pigments, off-flavours, odors, toxins, or other impurities. It also plays an important role in purifying proteins, enzymes, and other high-value food ingredients. Materials such as activated carbon, silica gel, and bentonite are frequently used as adsorbents due to their large surface area and high affinity for various molecules.
Example:
Unwanted flavours and colour compounds can be removed from vegetable oils by treating them with adsorbents like activated charcoal or bentonite, improving the oil’s sensory quality and stability.
Expert Tips: Components of a mixture interact with a solid adsorbent (stationary phase) and a liquid mobile phase. Separation occurs because each component adsorbs to the stationary phase with different strengths.
Partition chromatography separates components based on their differing polarities and solubilities in two immiscible phases: a stationary liquid phase coated onto a solid support and a mobile liquid phase that flows through the column. Compounds distribute themselves between the two phases depending on their polarity and solubility, leading to separation as they migrate at different rates.
Application
This method is especially useful for isolating lipid-soluble (non-polar) compounds from aqueous mixtures. It is applied in the analysis and purification of flavor compounds, essential oils, aroma chemicals, pigments, and other hydrophobic food constituents.
Example:
Essential oils or specific flavour molecules can be separated from complex food matrices using partition chromatography, enabling the extraction and purification of high-value aromatic compounds.
Expert Tips: Components of a mixture distribute between two immiscible phases: a solid stationary phase coated with liquid, and a liquid mobile phase. Separation occurs because each component partitions differently between the two phases.
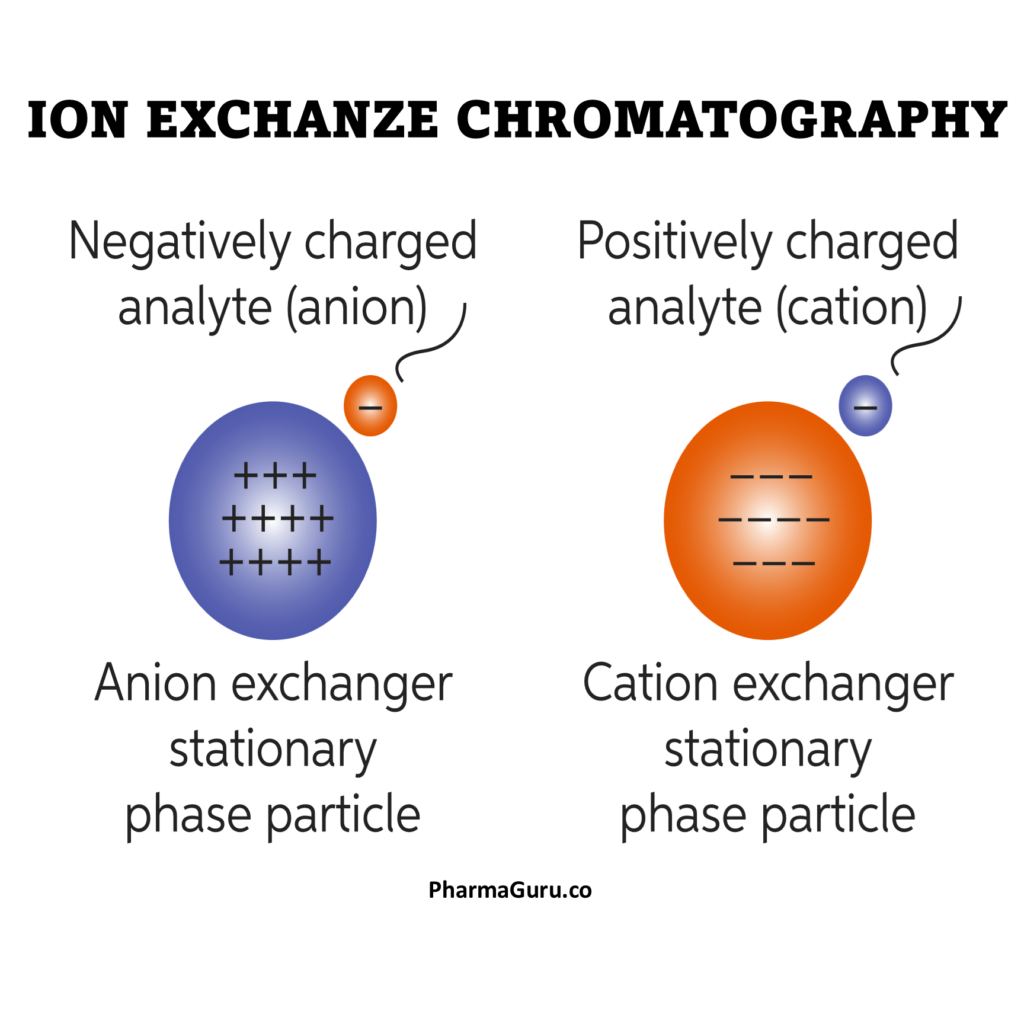
Ion exchange chromatography separates molecules based on their net electrical charge. The column is packed with an ion-exchange resin—either cation-exchange (negatively charged resin that binds positively charged molecules) or anion-exchange (positively charged resin that binds negatively charged molecules). Molecules with stronger opposite charges bind more tightly, while weaker ones elute earlier, enabling precise separation.
Application: This technique is widely used for the purification of proteins, peptides, and amino acids, as well as for desalting solutions, removing unwanted ions, and improving product quality. It also plays a significant role in water softening, sugar refining, and the purification of bioactive compounds.
Example: Ion exchange chromatography is commonly used to purify amino acids and proteins in dairy products, such as isolating whey proteins with high purity.
Expert Tips: Components of a mixture interact with a charged solid stationary phase (cationic or anionic) and a liquid mobile phase. Separation occurs based on differences in ionic interactions between the analytes and the ion-exchange sites on the stationary phase.
Size exclusion chromatography—also known as gel filtration chromatography—separates molecules based purely on size (molecular weight). A porous gel matrix is used as the stationary phase.
Application
SEC is used for the purification and characterisation of proteins, enzymes, polysaccharides, and food polymers. It allows the removal of aggregates or small molecules and helps determine molecular size distributions in food ingredients.
Example:
SEC can be used to separate polysaccharides, peptides, or protein fractions in processed foods, such as isolating specific molecular-weight components from plant or dairy protein extracts.
| HPLC Type | Separation Principle | Stationary Phase | Mobile Phase | Separates Based On |
|---|---|---|---|---|
| Partition HPLC | Differential partitioning of analytes between stationary and mobile phases | Chemically bonded phases (e.g., C18, C8; polar or non-polar surfaces) | Polarity/solubility differences | Reversible ion exchange between charged analytes and oppositely charged groups on the stationary phase |
| Adsorption HPLC | Adsorption–desorption interactions on the surface of the stationary phase | Polar solid surfaces (e.g., silica, alumina) | Non-polar organic solvents | Surface affinity, polarity, and strength of adsorption |
| Ion-Exchange HPLC | Reversible ion exchange between charged analytes and oppositely charged groups on stationary phase | Charged resins (cation/anion exchangers) | Buffered aqueous mobile phases | Charge and ionic interaction strength |
| Size-Exclusion HPLC | The liquid phase of opposite polarity to the stationary phase | Inert porous polymers or silica gels | Aqueous or organic solvents (non-interactive) | The liquid phase of opposite polarity to stationary phase |
Boost your pharma career with PharmaGuru’s expert-led online courses.: Online Pharma Course (Training)
Conclusion:
Overall, each chromatography technique separates molecules through a distinct mechanism—solubility (partition), surface binding (adsorption), charge interactions (ion exchange), or molecular size (size exclusion)—allowing analysts to choose the most effective method based on the specific properties of the compounds being purified.
You May Like
Further reading
Quick Links