Fourier Transform Infrared (FTIR) Spectroscopy is an analytical technique used to identify and analyze materials by measuring their absorption of infrared light. When IR radiation passes through a sample, its molecules absorb specific frequencies corresponding to their vibrational modes, producing a unique “molecular fingerprint” spectrum. This spectrum enables both qualitative and quantitative analysis of organic, […]
Fourier Transform Infrared (FTIR) Spectroscopy is an analytical technique used to identify and analyze materials by measuring their absorption of infrared light. When IR radiation passes through a sample, its molecules absorb specific frequencies corresponding to their vibrational modes, producing a unique “molecular fingerprint” spectrum. This spectrum enables both qualitative and quantitative analysis of organic, polymeric, and certain inorganic compounds.
In the pharmaceutical industry, FTIR plays a crucial role in every stage of development and production — from raw material identification and reaction monitoring to process control, API characterisation, and dosage form analysis. It is a fundamental tool used across all pharmaceutical laboratories for ensuring quality and compliance.
In this article, I’ll share my practical, skill-based insights on FTIR spectroscopy — covering its principles, applications, procedures, advantages, case studies, and frequently asked questions. By the end, this post will significantly enhance your understanding and practical knowledge of FTIR spectroscopy.
Boost your pharma career with PharmaGuru’s expert-led online courses.: Online Pharma Course (Training)
The range of IR is 12800 to 10cm-1 and can be divided into:
The mid-infrared region is used for IR studies.
IR spectroscopy studies interactions between Infrared radiation and matter.
Due to internal electronic rearrangement, atoms in the molecule do not remain fixed at their position but continuously vibrate at specific frequencies and produce IR radiation:
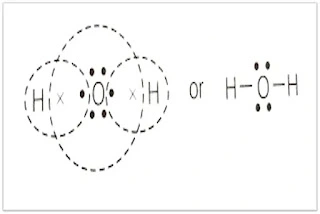
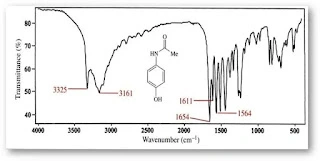
That vibrational frequency falls under the IR region. When IR radiation passes through the sample, it triggers the vibration of specific molecular bonds, and specific frequencies of IR radiation are absorbed. These same absorbed specific frequencies are missing from the transmitted light.
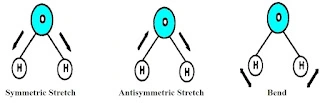
These absorbed frequencies lead to symmetric stretching, anti-symmetric stretching and bending-like vibration in the molecule:
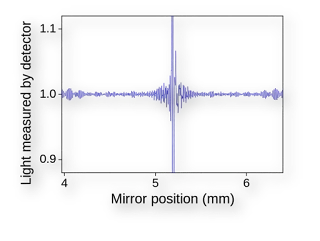
The centre of FTIR is the Michael interferometer, and it contains:
Related: Raman spectroscopy Vs FTIR Spectroscopy: Key Differences With FAQs
There are several procedures for preparing the sample for FTIR analysis but the following procedures are widely used in the industries:
Place about 5 mg of the sample and 500mg of KBr (potassium Bromide) in mortal. Grind properly using a pestle and make the pellet. Insert the pellet in a sample holder for FTIR analysis.
Place about 5 mg of the sample and one drop of Carbon tetrachloride.
Note:
The following procedures are adopted while performing FTIR:
Different functional groups give IR peaks at different wavenumbers. FTIR identifies these different functional groups. The below-mentioned molecule contains -OH(hydroxyl group), -NH-(amino group) and -CO- function groups:

Fourier Transform Infrared (FTIR) spectroscopy is one of the most powerful techniques used to identify chemical compounds and study molecular structures. The accuracy of FTIR results depends heavily on how the sample is prepared, and that’s where potassium bromide (KBr) and carbon tetrachloride (CCl₄) play key roles.
For solid samples, KBr pellets are the most common medium used in FTIR analysis. Here’s why:
In practice, a few milligrams of the powdered sample are blended with dry KBr and pressed under high pressure to form a thin, transparent pellet for measurement.
For liquid samples, carbon tetrachloride (CCl₄) is often used as a solvent. Its advantages include:
By using CCl₄, liquid samples can be analysed in a liquid cell with IR-transparent windows such as NaCl or KBr plates.
| Medium | Sample Type | Key Feature | Purpose |
|---|---|---|---|
| KBr | Solid | IR-transparent and inert | Forms pellets for solid FTIR samples |
| CCl₄ | Liquid | IR-transparent and non-polar | Solvent for liquid FTIR samples |
KBr provides an ideal, transparent medium for solids, while CCl₄ serves as a reliable solvent for liquids. Both ensure that the infrared light interacts primarily with the sample itself—revealing its true molecular fingerprint.
FTIR is widely used for Chemical Identification, Material Characterisation, Surface Analysis, Qualitative Analysis and Quantitative Analysis in the following industries:
It is like a chemical fingerprint. As the thumbs of two persons cannot match in the same way IR spectra of two different molecules can not match.
FTIR plays a crucial role in pharmaceutical analysis, offering rapid, reliable identification and quantification of drug substances and excipients. Its widespread acceptance by regulatory agencies further strengthens its value in both method development and routine quality control. With this understanding, you should now feel confident in applying FTIR techniques effectively. For further learning, you may also explore related topics such as FTIR calibration and the industrial applications of UV–Visible spectrophotometry.
You May Like
Fourier Transform Infrared (FTIR) Spectroscopy is an analytical technique used to identify and quantify chemical compounds by measuring how a sample absorbs infrared (IR) radiation. Each molecule absorbs specific IR frequencies corresponding to the vibrations of its chemical bonds, producing a unique spectrum — often called its molecular fingerprint.
The principle of FTIR is based on the absorption of infrared radiation by molecular vibrations. When IR light passes through or reflects off a sample, certain frequencies are absorbed due to molecular bond vibrations (stretching, bending, twisting). These absorbed frequencies are detected and mathematically converted using a Fourier Transform into a spectrum showing intensity versus wavenumber.
An FTIR spectrometer typically consists of the following main components:
Infrared Source: Emits broad-spectrum IR radiation.
Interferometer (Michelson type): Splits and recombines the IR beam to create an interference pattern.
Sample Holder/Compartment: Holds the sample in solid, liquid, or gas form.
Detector: Measures the transmitted or reflected IR light and converts it to an electrical signal.
Computer System: Performs the Fourier Transform and generates the final IR spectrum.
The fingerprint region lies between 1500–400 cm⁻¹ in the IR spectrum. It contains complex absorption bands unique to each molecule, much like human fingerprints. This region is mainly used for identification and structural confirmation of compounds.
Functional Group Region (4000–1500 cm⁻¹): Shows distinct peaks corresponding to major functional groups (e.g., –OH, –NH, –CH, –C=O).
Fingerprint Region (1500–400 cm⁻¹): Contains complex, overlapping vibrations specific to the molecule’s structure and bonding pattern.
Expert Tips: In short, the functional group region helps identify types of bonds, while the fingerprint region confirms the exact identity of the compound.
FTIR is widely used for:
Identification of organic and inorganic compounds
Purity testing and detection of contaminants
Quantitative analysis of mixtures
Reaction monitoring in chemical and pharmaceutical processes
Polymer and material characterisation
Verification of raw materials and finished products
The spectrometer passes a beam of infrared light through an interferometer, which modulates the light. The modulated beam then interacts with the sample, and the transmitted or reflected light reaches the detector. The resulting signal, called an interferogram, is processed using a Fourier Transform algorithm to produce the final IR spectrum — a plot of absorbance vs. wavenumber.
Rapid and non-destructive technique
High signal-to-noise ratio and excellent sensitivity
Requires minimal sample preparation
Can analyse solids, liquids, and gases
Provides both qualitative and quantitative information
Highly reproducible and accurate
ATR (Attenuated Total Reflectance) is a sampling technique used in FTIR to analyse samples without complex preparation. The IR beam passes through an optically dense crystal (like diamond or ZnSe) with a high refractive index. The light undergoes total internal reflection, and a small portion (the evanescent wave) penetrates the sample surface, allowing direct analysis of solids, liquids, or pastes.
FTIR detects O–H stretching vibrations around 3600–3200 cm⁻¹ and H–O–H bending around 1650 cm⁻¹. The presence, intensity, and shift of these bands indicate the type and amount of crystal water or hydration state in a compound, helping to distinguish between anhydrous and hydrated forms.
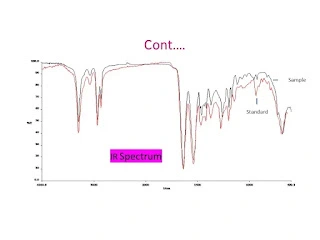
In the Simethicone assay, the FTIR technique measures the Si–O–Si stretching vibrations (typically near 1020–1100 cm⁻¹) and Si–CH₃ bands around 1260 cm⁻¹. By comparing the absorbance of these characteristic peaks against a standard reference spectrum, the concentration and identity of Simethicone in the sample are determined.
The identification test involves recording the IR spectrum of a sample and comparing it to a reference spectrum (from a pharmaopeia or standard library). A match in major absorption peaks and overall spectral pattern confirms the sample’s identity.
Further reading:
Quick Links