Both Assay and Potency play a vital role in controlling the quality, safety and efficacy of a pharmaceutical. Assay is the quantitative relative content of a drug, while potency is the quantitative absolute value of a drug standard Assay and Potency Assay The Assay is the quantitative relative content of a pharmaceutical. It is a […]
Both Assay and Potency play a vital role in controlling the quality, safety and efficacy of a pharmaceutical. Assay is the quantitative relative content of a drug, while potency is the quantitative absolute value of a drug standard
The Assay is the quantitative relative content of a pharmaceutical. It is a relative value and it is calculated against its corresponding standard while using a chromatographic technique or a spectroscopic technique. The potency of the standard is required in the assay calculation while using the chromatographic technique or spectroscopic technique.
The Assay can also be calculated using the chemical titration technique and in this technique, there is no need of any external standard. Since the chemical technique is not specific and that is why it is rarely used in the pharmaceutical industries.
Potency refers to the precise quantitative concentration of the active substance in a drug, or its intermediate forms, and is typically measured against established standards. The potency is calculated using the highest purity of the material, ensuring that all potential impurities—whether organic, inorganic, residual solvents, counterions, or water content—are accounted for during the determination process. This comprehensive approach ensures that potency reflects the true strength of the substance, considering all factors that could affect its efficacy.
| Potency | Assay |
| It is a quantitative value of the standard | It is a quantitative value of a drug substance or a molecule |
| It is the absolute value | It is a relative value |
| Calculated from chromatographic purity (while using the chromatographic technique) | It is calculated against the standard |
| Potency depends upon purity LOD/water, ROI or sulphated ash, inorganic impurities, counter ions, residual solvents etc. | Assay depends upon potency ( while using spectroscopic and chromatographic techniques). |
Potency is calculated from chromatographic purity using the following formula:
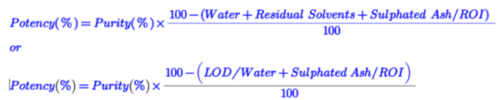
Assay is calculated against standard by external standard method or internal standard method using the following formula:
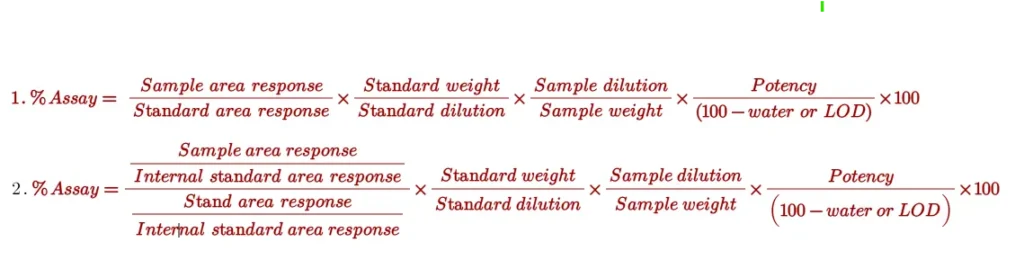
Assay of paracetamol is 99.7%: It indicates the relative content of paracetamol when calculated against paracetamol reference or working standards
Potency of paracetamol standard is 99.9%: It indicates the quantitative absolute value of paracetamol in the paracetamol standard
Related
Assay is the quantitative relative content of a drug, while potency is the quantitative absolute value of a drug standard
Further Reading
Quick Links