Learn how to evaluate linearity and range in method validation with step-by-step procedures, a real-life case study, and answers to common FAQs. Gain the confidence to perform the test independently.
Linearity and range in method validation are critical parameters, as they determine the concentration span over which accurate, precise, and reliable results can be obtained. Establishing these parameters ensures that the analytical method can effectively quantify the analyte across the intended range of concentrations. In this article, I will walk you through the procedure for evaluating linearity and range, supported by a detailed case study and a set of frequently asked questions (FAQs). By the end, you’ll have a clear understanding of the concept and be equipped to carry out the test confidently and independently.
Prepare two stock solutions A and B. Now prepare five solutions in the concentration range of 50% to 150% using stock solution A and B. Inject each of these five solutions (once) and generate the chromatogram. Plot the linearity graph using concentrations in the X axis and their corresponding area responses in the Y axis. Calculate correlation coefficient (R2) and slope. R2 should be more than or equal to 0.997
You may like:
A drug substance D having the following specifications for related substances:
Sample concentration is 1.0mg/ml to perform related substances test and QL of the method is 0.05%
The following concentrations (≥5) can be prepared to perform linearity:
The level of linearity and value of impurity A and their corresponding concertation have been calculated in the following table
| Level (L) | Impurity value (I) (0.20 x L/100 ) | Impurity solution concentration ( 1000 x I/100) |
| QL(0.05%) | 0.05% | 0.5mcg/ml |
| 50% | 0.10% | 1.0mcg/ml |
| 70% | 0.14% | 1.4mcg/ml |
| 100% | 0.20% | 2.0mcg/ml |
| 130% | 0.26% | 2.6mcg/ml |
| 150% | 0.30% | 3.0mcg/ml |
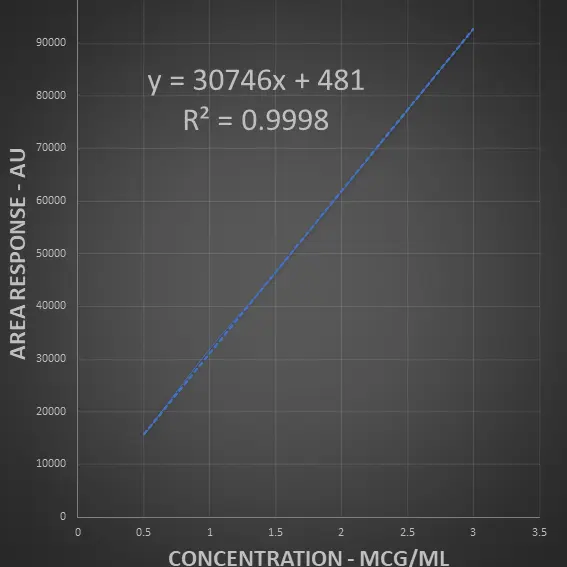
Inject Impurity A solution at each concentration as given in the above table and generate the chromatogram and note down the area response
| Impurity A ( mcg/ml) | Area response |
| 0.5 | 15457 |
| 1.0 | 31904 |
| 1.4 | 43400 |
| 2.0 | 61830 |
| 2.6 | 80380 |
| 3.0 | 92750 |
| Slope | 30746 |
| Correlation coefficient (R2) | 0.9993 ≥ 0.997 |
Acceptance Criteria: R2 is 0.9993 ≥ 0.997 and it passes the linearity criteria
Impurity A is linear between 0.05% to 0.30% (QL to 150% of the specification limit).
The difference between linearity and range in analytical method validation is fundamental yet often misunderstood. The following are the main differences between linearity and range:
Linearity
Definition:
Linearity refers to the ability of an analytical method to produce results that are directly proportional to the concentration of the analyte within a given range.
Key Points:
Range
Definition:
Range is the interval between the upper and lower concentration levels of analyte (including these levels) for which the method has been demonstrated to have suitable precision, accuracy, and linearity.
Key Points:
The following of linearity are widely used in pharmaceutical analysis:

Linearity and range are a key parameters in analytical method validation, as it defines the and concentration range over which the method produces accurate and precise results. Establishing linearity ensures the reliability of your analytical method across its intended application. If you have any suggestions or feedback regarding this article, feel free to share them in the comments section. For further assistance or inquiries, you can reach out through the contact form.
You may also want to check out other articles on my blog, such as:
To perform linearity and range assessment in method validation, begin by preparing a detailed protocol, selecting appropriate calibration standards, and analyzing samples across a defined concentration range. Evaluate the resulting data to establish the method’s linearity and determine the accurate and reproducible range
Linearity shows how well the method performs across concentrations (the quality of the relationship), whereas Range defines where the method performs well (the span of usable concentrations)
Disclaimer: The numerical data used in the tables or calculations are not actual data. It is designed to explain the topic.
Quick Links