Explore how DSC aids drug development by analyzing thermal behavior, stability, and polymorphism
Differential Scanning Calorimetry (DSC) is a thermal analysis technique widely used in drug development to study the thermal behaviour of substances, especially active pharmaceutical ingredients (APIs) and their interactions with excipients. It plays a crucial role in characterising materials, evaluating stability, and identifying potential formulation or compatibility issues early in the development process.
Understanding the thermal behaviour of drug substances and formulations is crucial for ensuring stability, efficacy, and safety during medicine development. One of the most powerful techniques used for this purpose is Differential Scanning Calorimetry (DSC). This thermal analysis method provides detailed information about the physical and chemical changes in drug compounds under controlled temperature conditions.
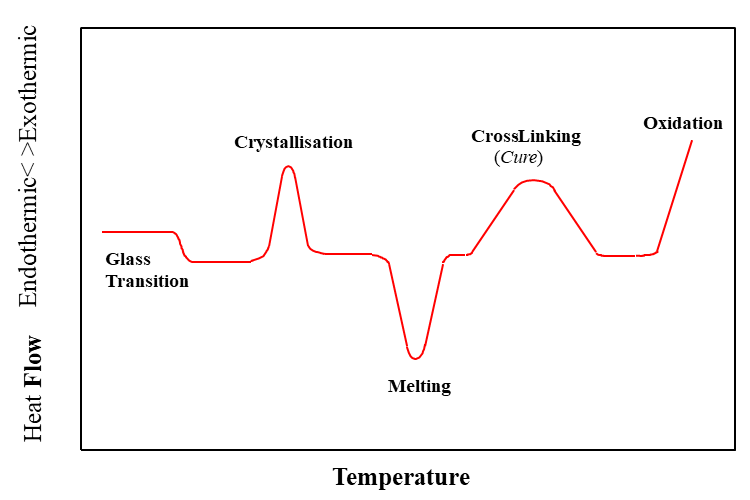
DSC is used in pharmaceuticals to study the thermal behaviour of drug substances and formulations. It helps determine melting points, glass transitions, polymorphism, stability, and compatibility with excipients, critical for drug development and quality control.
Purity by DSC refers to thermal purity, estimated based on melting point depression caused by impurities, typically useful for assessing crystalline substances. It provides insight into physical purity, but it is an approximation and assumes ideal behaviour.
Chemical purity, on the other hand, is measured using techniques like HPLC or titration and quantifies the actual chemical composition, detecting both organic and inorganic impurities, regardless of their thermal behaviour.
DSC is used to measure the heat flow associated with material transitions such as melting, crystallization, and glass transitions.
The main purpose of DSC is to study thermal properties of materials, like heat capacity, phase transitions, and stability.
In pharmaceuticals, DSC is used to analyze drug stability, polymorphism, formulation, and excipient compatibility.
DSC is a thermal analysis technique that measures the difference in heat flow between a sample and a reference material as they are heated or cooled. This helps to identify thermal events like melting or crystallization.
Advantages include high sensitivity, precise measurement of phase transitions, ability to analyze small sample sizes, and fast results.
DSC qualification involves ensuring that the instrument is properly calibrated and validated to provide accurate, reproducible data.
Limitations include its inability to detect non-thermal changes, sample size limitations, and possible interference from volatile substances.
The principle of DSC is based on measuring the heat flow difference between a sample and a reference as they undergo temperature changes, allowing the detection of thermal transitions in the material.
Differential Scanning Calorimetry (DSC) is a thermoanalytical technique used to measure the heat flow associated with phase transitions of a material as a function of temperature or time. It helps identify critical properties such as melting point, glass transition temperature, crystallisation, and purity—key factors in pharmaceutical formulation and processing.
The principle of DSC is based on the measurement of heat flow differences between a sample and an inert reference as both are subjected to the same controlled temperature program.
The basic steps in a DSC analysis are as follows:
A DSC thermograph is typically a 2D plot with the following axes:
| Thermal Event | Graph Representation | Direction |
|---|---|---|
| Melting Point (Tm) | Sharp endothermic peak | Downward (usually) |
| Glass Transition (Tg) | Step change in baseline (no peak) | Downward |
| Crystallization | Exothermic peak (if re-crystallizing upon cooling) | Upward |
| Decomposition | Broad, exothermic or endothermic events | Variable |
How to Draw or Label It:
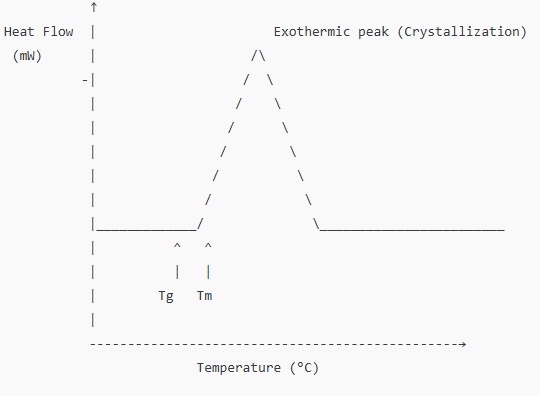
Background:
Carbamazepine, an anticonvulsant drug, exhibits polymorphism, existing in multiple crystalline forms. These forms can have significantly different solubility, stability, and bioavailability.
DSC Application:
Researchers used DSC to identify and distinguish between different polymorphs based on their melting points and enthalpies of fusion.
Findings:
The distinct thermal signatures enabled scientists to monitor polymorphic transitions during manufacturing and storage, ensuring consistency in the final product.
DSC is widely applied across different stages of drug development:
While DSC is highly useful, it does have some limitations:
| Limitation | Explanation |
|---|---|
| Low sensitivity for trace events | Minor thermal events may go undetected without sample concentration adjustments. |
| Overlapping transitions | Closely spaced thermal events may be difficult to resolve. |
| Small sample size | Limits representativeness for heterogeneous samples. |
| Interpretation requires expertise | Data analysis can be complex and may need complementary techniques (e.g., XRD). |
Differential Scanning Calorimetry (DSC) plays an indispensable role in modern drug development. By providing insights into thermal behaviours, phase transitions, and material compatibility, DSC supports informed decisions during formulation, stability testing, and quality assurance. While it has certain limitations, its strengths make it a staple in pharmaceutical thermal analysis and material characterisation.
You May Like:
Further Reading
Quick Links