Learn Starting Material and Key Starting Material, definition, key differences, and case studies with FAQs
Starting Material and Key Starting Material seem similar, but they have distinct definitions and roles in the pharmaceutical development process.
Starting Material (SM) and Key Starting Material (KSM) terms often cause confusion while selecting the ROS (route of synthesis) during pharmaceutical development. Whether you are in the early stages of drug development or refining an existing manufacturing process, taking the time to understand and correctly identify starting materials and KSMs is an essential part of ensuring the success of your pharmaceutical product. That is why I decided to share my skill-based knowledge on this topic. In this blog, I will discuss what each term means, highlight the key differences, and use a case study to demonstrate their real-world application.

Major Takeaway: FAQs
Key Starting Materials (KSMs) are critical substances in the manufacturing of an Active Pharmaceutical Ingredient (API) that directly influence the final product’s chemical structure, quality, and safety. These materials are closely monitored and regulated due to their significant role in the synthesis process, and they often have a direct impact on the purity and consistency of the final drug. Examples include specific chemicals or intermediates that play a pivotal role in the formation of the API.
A starting material is any raw substance or ingredient used in the manufacturing process of an Active Pharmaceutical Ingredient (API) or drug product. These materials can include chemicals, excipients, solvents, or intermediates, and serve as the foundational components from which the final drug is synthesized. Starting materials may not always directly impact the drug’s final chemical structure or quality, unlike Key Starting Materials (KSMs).
A starting material is any raw substance used in drug manufacturing, while a key starting material is a critical component that directly influences the final product’s structure, quality, and regulatory compliance.
A starting material is any raw substance used in drug manufacturing, while a key starting material is a critical component that directly influences the final product’s structure, quality, and regulatory compliance.
Starting materials (SM) refer to the raw ingredients or substances that are used to synthesise or manufacture an active pharmaceutical ingredient (API) or drug product. These materials can be chemicals, solvents, intermediates, or biological materials. They are the fundamental components from which the API is derived.
In the pharmaceutical industry, the quality, purity, and source of starting materials are extremely important as they directly affect the final drug’s safety, efficacy, and consistency.
A Key Starting Material (KSM) is a specific starting material that is critical in the synthesis of the API. It is often a substance that directly contributes to the chemical structure of the final drug. KSMs are generally identified based on their role in the manufacturing process, particularly if they have the potential to impact the final product’s quality, safety, and compliance.
You May Like
The key factors that determine whether a material is considered a Key Starting Material include:
| Aspect | Starting Materials (SM) | Key Starting Materials (KSM) |
|---|---|---|
| Definition | Raw materials used in the manufacturing of an API or drug product. | A critical starting material directly affecting the synthesis and quality of the API. |
| Role in Synthesis | Used to produce the final API or drug product, but not necessarily essential to the core chemical structure. | Plays a pivotal role in defining the chemical structure and properties of the API. |
| Regulatory Focus | Standard control and documentation. | More stringent regulatory control and traceability required. |
| Impact on Final Product | Indirect or less direct impact on the quality of the final API. | Direct impact on the quality, safety, and effectiveness of the API. |
| Examples | Phenol in acetaminophen synthesis | 4-Nitrophenol in acetaminophen synthesi |
To bring our understanding of starting materials and KSMs into context, let’s take a look at the production of Paracetamol (Acetaminophen).
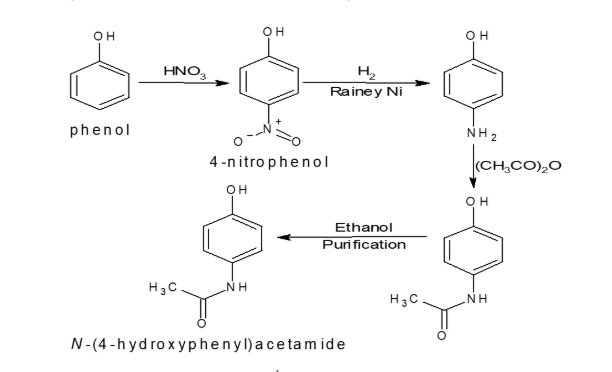
The Synthesis Process:
Paracetamol is one of the most commonly used pain relievers worldwide. The synthesis involves several chemical reactions starting with simple raw materials. Let’s break it down:
A key precursor to 4-Aminophenol is 4-Nitrophenol, which undergoes a reduction reaction to form 4-Aminophenol. However, during the reduction process, there is a possibility of forming different isomers at the 4-Nitrophenol stage. Given its critical role in determining the final product’s structure and purity, 4-Nitrophenol is considered a Key Starting Material (KSM). Its significance lies in the potential for isomeric variations, making it essential for rigorous control and monitoring in the manufacturing process.
The pharmaceutical industry must meet stringent guidelines when it comes to the handling of KSMs. For instance, both the FDA and EMA often mandate that KSMs be clearly identified in the regulatory submission process. Manufacturers must provide detailed information about the source, quality control, and any potential impurities associated with KSMs, as they can impact the therapeutic quality of the API.
The distinction between Starting Materials and Key Starting Materials is fundamental in pharmaceutical development. While both are essential to producing high-quality APIs, KSMs have a more direct and significant impact on the final product, and their regulatory scrutiny is greater. By understanding these materials and their roles, pharmaceutical developers can ensure that their products are safe, effective, and compliant with global standards.
In pharmaceutical production, where precision is paramount, knowing the difference between these terms can make a real difference in manufacturing, quality control, and regulatory compliance.
Further Reading
Quick Links