
PharmaGuru Premium Pharmaceutical Training programs are designed to help students, freshers, and working professionals build industry-ready skills, gain practical exposure, and accelerate their pharma careers.
Whether you are just starting or aiming for advanced expertise, PharmaGuru has the right course at the right stage of your career.
Table of Contents
PharmaGuru Premium Training Categories
The premium courses come under the following three categories:
- Every Pharma Professional Foundation Course (course fee: Just ₹199)
- PharmaGuru Most Popular Courses (course fee: ₹8100)
- PharmaGuru Advanced Courses (course fee: ₹16200)
Note: All courses are best suited for beginners and professionals. Choose the right course as per your convenience and start learning today
Every Pharma Professional – Foundation Course
Course Fee: Just ₹199
Start Your Pharma Career Journey With Clarity and Confidence: Just ₹199
This foundation-level course is the perfect entry point for anyone looking to understand the pharmaceutical industry, analytical basics, and career pathways.
Why Choose This Course?
- Ideal starting point before advanced PharmaGuru programs
- Covers industry expectations & basic analytical concepts
- Extremely affordable with high practical value
- Designed to boost awareness and confidence
Best For:
Students | Freshers | Career switchers | Early professionals
Outcome:
- Strong foundation
- Clear career direction
- Better decision-making for advanced learning
👉 Enroll Now & Start Learning – Just ₹199
PharmaGuru Most Popular Courses
Course Fee: ₹8,100
Build Practical, Job-Oriented Pharma Skills
These courses focus on hands-on training required for real-world pharma roles, especially in QC, QA, Analytical Research, and R&D.
Key Highlights:
- Industry-oriented & practical learning
- Interview-focused preparation
- Strong foundation for pharma job roles
- Ideal bridge between basics and advanced expertise
👉 Buy Now & Start Learning – Just ₹8100
PharmaGuru Advanced Courses
Course Fee: ₹16,200
Accelerate Your Career with Advanced Pharma Expertise
Advanced programs designed for working professionals and serious learners aiming for technical mastery, specialization, and leadership roles.
What You’ll Gain:
- Advanced analytical & research concepts
- Method development & validation expertise
- QbD-based practical applications
- Long-term career growth & specialization
👉 Enroll Now – Just ₹16,200
Note: Enrollment and payment can also be completed via the “Enroll Now” button.
Course Comparison: Every Pharma Professional vs Most Popular vs Advanced
| Feature | Every Pharma Professional | Most Popular Courses | Advanced Courses |
|---|---|---|---|
| Level | Beginner | Intermediate | Advanced |
| Target Audience | Students / Freshers | Analytical / QC / QA / RA / R&D | Industry Professionals |
| Purpose | Career orientation | Skill development | Career acceleration |
| Practical Depth | Basic | Moderate to High | Advanced |
| Best Use | Entry gateway | Job readiness | Expertise & growth |
| Total Sessions | 2 | 5 | 12 |
| Total Duration | 2 Hours | 7.5 Hours | 18 Hours |
| Course Fee | ₹199 | ₹8,100 | ₹16,200 |
| Professional Support | 6 Months | 1 Year | 5 Years |
| Content Level | Basic | Basic to Advanced | Basic to Subject Matter Expert |
| Certificate | Yes | Yes | Yes |
| Downloadable Materials | Yes | Yes | Yes |
| Money-Back Guarantee | Yes | 30 Days | 60 Days |
| Secure Payment Gateway | Razorpay | Razorpay | Razorpay |
👉 Start with “Every Pharma Professional” and upgrade anytime as your skills grow.
Course List | Popular & Advanced
We currently offer 7 high-demand pharma training programs designed to align with industry needs:
- Ultimate Chiral Separation Training by HPLC & GC: From Basics to Advanced
- The High-Demand HPLC Method Development Training: Basics to Advanced
- Mastering Analytical Method Validation: Complete Training from Basics to Advanced
- QbD-Based Pharmaceutical Impurity Control Training: From Basics to Advanced
- Mastering Pharmaceutical Stability & Shelf Life Evaluation
- QbD-Based Analytical Control Preparation Training For Pharma Professionals
- Step-by-Step GC Method Development Training
Courses List | Every Pharma Professional (Just ₹199)
Explore 27+ foundation-level courses covering essential pharma concepts:
- HPLC For Every Pharma Professional
- GC For Every Pharma Professional
- Titration For Every Pharma Professional
- LC-MS For Every Pharma Professional
- GC-MS For Every Pharma Professional
- GCHS For Every Pharma Professional
- HPLC Mobile Phase Chemistry
- HPLC Column Chemistry and Column Care
- QBD-Based Forced Degradation Study
- Photo Stability Studies
- Nitrosamine Impurities: Challenges & Solutions
- Carriers in Pharmaceutical Industries
- RFT-Based Technology Transfer of Analytical Method
- GLP For Every Pharma Professional
- How To Calibrate HPLC & GC?
- FTIR Technique & UV-Vis Spectrophotometer
- OOS and OOT: How To Manage?
- SOP, Protocol & Report | GDP
- Effective CAPA Management
- 21CFR Compliance
- STP: Effective Preparation
- QC Monograph Preparation
- How to perform an Internal audit?
- Analytical Literature Report: How to prepare?
- Analytical Solution Preparation for HPLC & GC
- How to design specifications for different pharmaceutical tests?
- How to decide System suitability testing SST) for HPLC and GC?
- KF (Karl Fisher) Technique and LOD techniques
- How to handle an FDA audit?
- Deficiency Letters management
- HPLC & GC Troubleshooting
- Chiral Method development
- …and many more
👉 Buy Now & Start Learning for Just ₹199
PharmaGuru Global Training Schedule
PharmaGuru offers globally accessible live trainings designed to suit pharma professionals across India, Asia, Europe, and the Americas.
For Every Pharma Professional
📅 Training Days
On 1st, 2nd and 3rd Sunday (in two slots)
🕒 Live Training Slots (India Time – IST)
- Slot 1: 7:30 PM – 9:00 PM IST (Ideal for India, Asia, Middle East & Europe)
- Slot 2: 10:00 PM – 11:30 PM IST (Ideal for Europe, North & South America)
PharmaGuru Most Popular
🕒 Live Training Slots (India Time – IST)
📅 Training Days
Live trainings are scheduled on Monday to Friday during the 1st, 2nd, and 3rd weeks of every month, with two available time slots each day.
- Slot 1: 7:30 PM – 9:00 PM IST (Ideal for India, Asia, Middle East & Europe)
- Slot 2: 10:00 PM – 11:30 PM IST (Ideal for Europe, North & South America)
PharmaGuru Advanced Courses
Note: Sessions are scheduled in one or both slots depending on course depth and target audience.
Global Accessibility
- All timings are optimized to work across major global time zones.
- Participants are encouraged to check their local time before joining.
📌 Additional Notes
- Live interactive sessions with expert trainers
- Recording access provided (as applicable)
- Schedule may vary slightly based on course and faculty availability
Why PharmaGuru?
- Beginner to Advanced structured learning
- Industry-relevant curriculum
- Affordable pricing with a money-back guarantee
- Long-term professional support
- Trusted by pharma students & professionals
Take the First Step Today
Choose the right PharmaGuru course and invest in your pharma career now.
Course Syllabus & Learning Outcomes
1. Ultimate Chiral Separation Training by HPLC & GC: From Basics to Advanced (PharmaGuru Training)
Module 1: Selecting the Right Chiral Separation Technique
- How to choose between Chiral HPLC and Chiral GC based on analyte properties, volatility, polarity, thermal stability, MW, and regulatory needs.
- Decision tree for technique selection with real-world examples.
Module 2: Principles of Chiral Method Development
- Fundamental concepts of enantioselectivity, chiral recognition, and asymmetric interactions.
- Key parameters: selectivity (α), resolution (Rs), efficiency (N), and retention (k’).
- Understanding three-point interaction models in chiral separations.
Module 3: Selecting Mobile Phase for Chiral HPLC
- How to choose normal-phase vs reversed-phase vs polar-organic modes.
- Rules for selecting heptane/alcohol ratios, aqueous buffers, and polar solvents.
- Mobile phase impact on selectivity, resolution, retention, and stability.
Module 4: Selecting Modifiers for Chiral HPLC
- Choosing the right alcohol, acid/base additives, and polar modifiers.
- How modifiers influence interaction mechanisms, peak shapes, and enantioselectivity.
- Troubleshooting issues caused by modifiers.
Module 5: Selecting the Right Chiral Column (CSP) for HPLC
- Overview of polysaccharide, Pirkle-type, cyclodextrin, protein, and macrocyclic antibiotic CSPs.
- How to select CSPs using structure-based screening and interaction mapping.
- How to design a column screening strategy to maximise success.
Module 6: Selecting CSPs for Chiral GC
- Choosing cyclodextrin-based, derivatised, and specialised GC chiral stationary phases.
- Selection based on volatility, functional groups, stability, and temperature limits.
- GC-specific chiral screening strategy.
Module 7: Structured Separation Strategies for Different Types of Chiral Molecules
a) Molecules with One Chiral Centre
- Predicting interactions and choosing the correct CSP and mobile phase.
- Simplified decision map for first-pass screening.
b) Molecules with Two Chiral Centres
- Understanding diastereomeric vs enantiomeric separation challenges.
- Strategy to handle multiple peaks and co-elution.
c) Molecules with Multiple Chiral Centres
- Handling complex stereochemical patterns.
- Advanced column pairing and multi-technique approaches.
Module 8: Reducing Analysis Cost in Chiral HPLC & GC
- Techniques to cut solvent usage, runtime, and column wear.
- How to select cost-efficient columns and mobile phases without sacrificing quality.
- Using common method strategies.
Module 9: Optimization & Finalization of Routine Chiral Methods
- Step-by-step optimisation workflow: screen → refine → optimise → validate.
- Robustness, intermediate precision, and system suitability criteria.
- Converting development methods into validated routine methods.
Module 10: Preparing a Chiral Method Development Report
- How to document:
- Column screening results
- Mobile phase rationale
- Optimization experiments
- System suitability justification
- Final method summary
- Regulatory expectations for pharma-quality chiral methods.
Module 11: Case Studies, FAQs & Real-World Problem Solving
- 7+ detailed case studies covering:
- One-centre and multi-centre chiral molecules
- HPLC vs GC technique decisions
- Troubleshooting poor selectivity, co-elution, and long runtime
- Cost reduction examples
- Interactive FAQs with practical troubleshooting guidance.
- Bonus: Real industry challenges and expert solutions.
2. The High-Demand HPLC Method Development Training: Basics to Advanced (PharmaGuru)

Module 1: Getting Started with HPLC Method Development
- How to initiate HPLC method development for assay, related substances, and impurity profiling.
- Understanding regulatory expectations for method scope and target performance.
Module 2: Principles of HPLC Separation Mechanism
- Fundamentals of chromatographic interactions, retention, selectivity, and resolution.
- Role of partitioning, adsorption, ion-exchange, and size exclusion in separation efficiency.
Module 3: Selecting the Right HPLC Mode
- Choosing between reversed-phase, normal-phase, ion-pair, HILIC, and ion-exchange modes.
- Mode selection based on analyte polarity, pKa, functional groups, solubility, and complexity.
Module 4: Selecting HPLC Columns / Stationary Phases
- How to choose the best C18, C8, phenyl, polar-embedded, HILIC, and specialty phases.
- Column selection workflow using analyte structure and separation goals.
- Column screening strategies to increase first-pass success.
Module 5: Method Development for Simple Molecules (<5 Impurities)
- Designing a fast, efficient, and cost-effective method for low-complexity impurity profiles.
- Approach for early screening, mobile phase optimisation, and resolution mapping.
Module 6: Method Development for Complex Molecules (>5 Impurities)
- Structured strategy for multi-impurity separation, including co-elution challenges.
- Gradient design, selectivity tuning, peak tracking, and system suitability mapping.
Module 7: Separating Impurities Along with Isomers & Diastereomers
- Advanced separation approaches for positional isomers, stereoisomers, and diastereomers.
- Use of pH, organic modifiers, and column chemistry to enhance selectivity.
- Real-case strategies for resolving closely eluting or overlapping peaks.
Module 8: Deciding System Suitability Criteria (SST)
- How to define meaningful SST parameters: Rs, tailing, RSD, plate count, retention time.
- Linking SST requirements to method intent and risk assessment.
Module 9: Impurity Calculations
- How to calculate individual impurities, total impurities, unknowns, and % area calculations.
- Relative response factors (RRF): when and how to apply them accurately.
- Acceptance criteria: regulatory expectations.
Module 10: Optimizing and Finalizing the Routine Method
- Step-by-step workflow: screening → optimisation → robustness → finalisation.
- Setting final gradients, buffer strengths, pH, flow rate, and column temperature.
- Ensuring method ruggedness for QC environments.
Module 11: Method Verification & Development Report Writing
- How to perform method verification, transfer checks, and suitability confirmation.
- Preparing a complete method development report with scientific justification and data summaries.
Module 12: Reducing HPLC Analysis Costs
- Strategies to lower the cost per analysis using:
- Shorter columns
- Reduced solvent usage
- High-efficiency columns
- Smart gradient design
- Approaches to reduce re-runs, downtime, and column damage.
Module 13: 11+ Real-World Case Studies & Expert FAQs
- Case studies covering:
- Simple and complex impurity profiles
- Isomer and diastereomer resolution
- SST troubleshooting
- Cost optimisation examples
- Real R&D and QC industry challenges
- Detailed FAQs with problem–solution formats.
- Bonus: pro tips for handling unexpected chromatographic behaviour
3. Mastering Analytical Method Validation: Complete Training from Basics to Advanced (PharmaGuru)
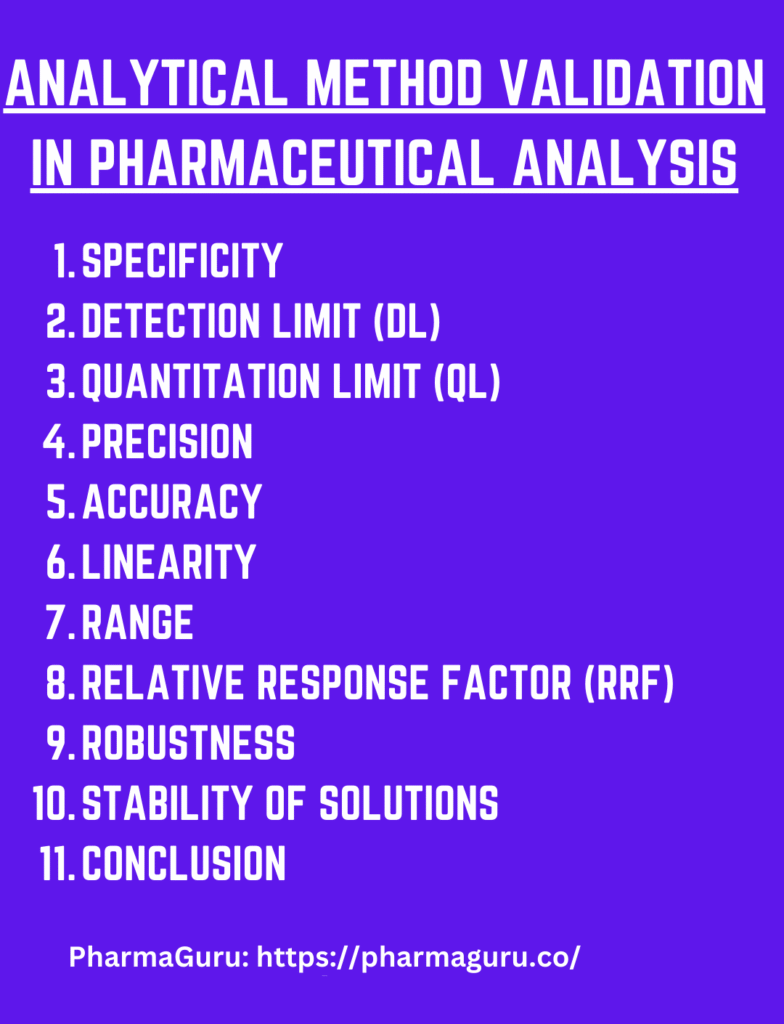
Method Validation Strategy
- Definition and regulatory importance of method validation
- Pre-validation checks and readiness assessments
- Method classification (assay, impurities, dissolution, content uniformity, etc.)
- How to design a scientifically justified validation protocol
- Protocol review, circulation, and approval process
Specificity
- Why is specificity required for reliable quantification
- How to demonstrate specificity using:
- Blank
- Placebo
- Known impurities
- Stress/degradation samples
- Assessing peak purity and interference
Precision
- How to perform system repeatability (system precision)
- How to evaluate method repeatability (intra-day precision)
- How to assess intermediate precision/reproducibility using different analysts, days, and instruments
- Acceptance criteria and interpretation
Detection Limit (DL)
- Different methods for determining DL:
- Visual evaluation
- Signal-to-noise method
- Standard deviation approach
- Acceptance criteria for DL
- Importance of DL in trace-level impurity detection
Quantitation Limit (QL)
- Approaches for determining QL:
- S/N method
- Standard deviation method
- Calibration curve method
- Acceptance criteria for QL
- Role of QL in routine impurity quantification and limit tests
Linearity, Range & Relative Response Factor (RRF)
- Why linearity and range are essential
- Designing linearity studies (levels, replicates, concentration span)
- Evaluating slope, intercept, correlation, residuals, and ANOVA
- When and how to establish RRF for impurities
- Acceptance criteria and troubleshooting
Accuracy & Recovery
- How to perform accuracy studies at different concentration levels
- Designing recovery studies for assay and impurity methods
- Acceptance criteria for accuracy data
- Managing nonlinearity or poor recovery behaviour
Robustness
- How to select robustness parameters (pH, flow, temperature, wavelength, column, mobile phase composition, etc.)
- One-factor-at-a-time vs multivariate approaches
- How to respond to robustness failures and redesign method parameters
Stability of Solutions
- Why is solution stability required
- Study design for sample, standard, and impurity solution stability
- Acceptance criteria and justification of solution hold times
Case Studies & Conclusion
- Multiple real-world case studies (assay, impurities, stability-indicating methods)
- Example of protocol creation, execution, and documentation
- How to prepare a complete validation report
- Approval and archival process
And Much More…
- Bonus tips, troubleshooting guides, common mistakes, regulatory insights, documentation tools, checklist templates, and additional expert recommendations
4. QbD-Based Pharmaceutical Impurity Control Training: From Basics to Advanced (PharmaGuru)
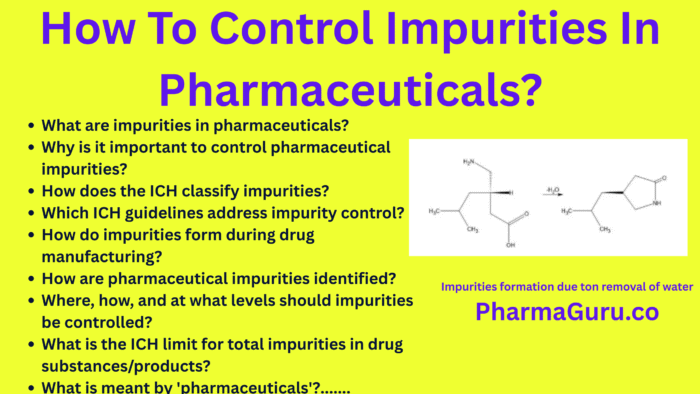
Control of Chiral Impurities
- Strategies for identification, quantification, and separation
- HPLC, GC, and other analytical techniques for chiral impurity monitoring
- Regulatory requirements and best practices
Control of Achiral Impurities
- Identification and monitoring of process-related and synthetic impurities
- Analytical approaches for assay and impurity profiling
- Acceptable limits and documentation
Control of Isomeric Impurities
- Understanding positional and stereoisomeric impurities
- Methods for separation and quantification
- Role of HPLC, LC-MS, and chiral columns in impurity control
Control of Degradation Impurities
- Forced degradation studies (acid/base, thermal, oxidative, hydrolytic)
- Photostability studies and light-sensitive degradation assessment
- Designing stability-indicating methods
Control of Genotoxic Impurities
- Identification and monitoring of mutagenic/genotoxic impurities
- Threshold of Toxicological Concern (TTC) and risk assessment strategies
- Analytical and computational approaches (SAS, in-silico)
Control of Nitrosamine Impurities
- Regulatory guidelines and risk assessment for nitrosamines
- Screening, quantification, and acceptable limits
- Mitigation strategies in manufacturing and formulation
Control of Elemental Impurities
- Understanding sources of elemental impurities (ICH Q3D)
- Techniques for detection (ICP-MS, ICP-OES)
- Setting limits, monitoring, and compliance
Impurity Control Strategy
- How, where, and at what levels impurities should be controlled
- Role of TTC, SAS, and in-silico systems in impurity risk assessment
- Purge factor studies to ensure process safety and regulatory compliance
Regulatory Guidelines
- ICH guidelines (Q3A, Q3B, Q3C, Q3D, M7)
- FDA, EMA, and other global regulatory requirements
- Documentation, reporting, and approval expectations
Case Studies & Practical Learning
- 11+ real-world case studies covering all impurity types
- Practical insights into analytical method design and risk mitigation
- Interview FAQs and expert tips for real-world scenarios
- And much more… bonus troubleshooting, regulatory tips, and best practices
5. Mastering Pharmaceutical Stability & Shelf Life Evaluation: Complete Training (PharmaGuru)
Initiating Stability Studies
- How to initiate stability studies for new products or APIs
- Selecting representative batches for study
- Designing a scientifically justified stability protocol
- Regulatory expectations and approval workflow
Understanding Stability vs Retest Period vs Shelf Life vs Holding Time
- Differences between stability studies, retest period, shelf life evaluation, and holding time studies
- How each impacts product release, shelf life labelling, and regulatory compliance
- Designing studies based on product type and regulatory guidelines
Principles of Stability Studies & Shelf Life Evaluation
- Fundamental concepts behind pharmaceutical stability
- Key factors affecting product degradation: chemical, physical, and microbiological
- Guidelines for establishing shelf life based on stability data
Real-Time and Accelerated Stability Correlation
- Designing and conducting real-time vs accelerated stability studies
- Extrapolating accelerated data to predict long-term stability
- Understanding ICH Q1A(R2) correlation requirements
Role of Photostability & Forced Degradation Studies
- Importance of photostability studies in regulatory compliance
- Conducting forced degradation studies to identify degradation pathways
- Integration of results into shelf life evaluation
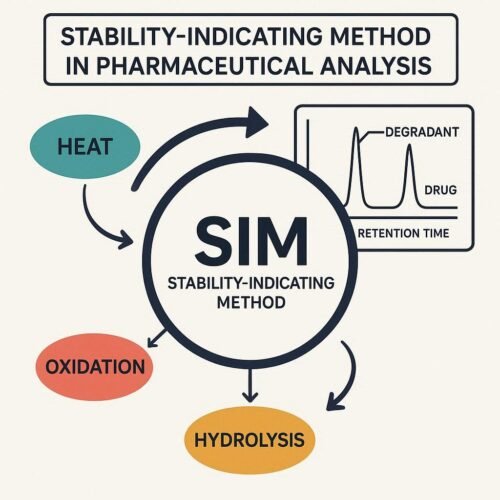
Role of Stability-Indicating Methods (SIM)
- How SIM supports accurate stability and shelf life evaluation
- Selection and validation of analytical methods for stability testing
- Monitoring degradation products, impurities, and potency over time
Container Closure & Packaging Interaction Studies
- Assessing the impact of packaging and container closure systems on stability
- Understanding extractables, leachables, and interaction studies
- Regulatory expectations and documentation best practices
Data Analysis, Trending & Out-of-Spec/Out-of-Trend (OOS/OOT)
- Techniques for data trending and statistical evaluation
- Investigating OOS and OOT results during stability studies
- Generating actionable insights and final reports
And Much More…
- Practical tips, case studies, troubleshooting, and expert recommendations for regulatory-compliant stability and shelf life evaluation
6. QbD-Based Analytical Control Preparation Training For Pharma Professionals (PharmaGuru)
Specifications Design Across Process Stages
- Designing analytical specifications for raw materials, reaction mixtures, intermediates, and final API
- Linking specifications to process understanding and product quality
- Regulatory expectations for stage-wise control
Trend Analysis & Purification Assessment
- Techniques to monitor trends in impurity levels across batches
- Evaluating purification efficiency at different stages
- Data-driven approaches for process optimisation
Control Strategies for Impurities
- Systematic strategies to control known and unknown impurities
- Setting critical limits and acceptance criteria
- Preventive measures and risk-based approaches
Identification & Characterisation of Unknown Impurities
- How to detect and isolate unknown impurities
- Structural characterisation using HPLC, GC, LC-MS, NMR, and other techniques
- Linking impurity identification to analytical and regulatory strategies
Impurity Control: Where, How, and at What Level
- Determining critical control points in the manufacturing process
- Establishing acceptable impurity levels based on risk assessment and regulatory guidelines
- Implementing stage-wise monitoring plans
Role of Cross-Functional Teams
- Collaborating across Analytical, QC, QA, RA, and R&D for robust control strategies
- Defining responsibilities and data ownership
- Leveraging team expertise for QbD-driven method and specification design
Applications of Analytical Control
- Applications in raw material testing, in-process monitoring, stability studies, and release testing
- Role in regulatory submissions and compliance
- Integration with PAT (Process Analytical Technology) and QbD approaches
And Much More…
- Practical case studies, troubleshooting strategies, regulatory tips, and expert insights
- Guidance on report preparation, documentation, and approval workflow
- Advanced techniques for impurity monitoring and control
7. Step-by-Step GC Method Development Training: From Fundamentals to Advanced Applications (PharmaGuru)

Comprehensive Step‑by‑Step GC Method Development Workflow
Learn the full systematic process — from defining analytical objectives to final documentation and STP preparation — for robust and reproducible GC methods.
Smart Selection of GC columns & Conditions
Master how to choose the right column, detector, carrier gas, diluent, and injection parameters tailored to your sample and target analytes.
Optimising Oven Temperature Programs
Understand how to develop and fine‑tune GC temperature programs that balance resolution, run time, and peak performance.
System Suitability & Method Validation Essentials
Learn how to set key SST criteria (resolution, plate count, tailing) and perform method verification/validation to ensure regulatory‑ready methods.
Practical Sample & Diluent Preparation Strategies
Discover best practices for sample prep — including dilution, filtration, and solvent choices — to avoid interferences and ensure reliable analysis.
11+ Real‑World Case Study Implementation
Apply your knowledge with practical GC case examples, such as separating solvents like methanol, ethanol, isopropyl alcohol, and toluene with optimised GC conditions.
Expert Tips on Troubleshooting & Optimisation
Get insider techniques to refine GC parameters (stationary phase polarity, film thickness, flow rates) for peak sharpening and improved separations.
FAQs: Premium Pharmaceutical Training
What is the Every Pharma Professional Foundation Course?
Every pharma professional foundation course is designed for every pharma student and professional to understand industry expectations, basic analytical concepts, and career pathways in the pharmaceutical industry
What is the Most Popular Course?
The Most Popular Course offers core pharmaceutical training, covering topics from basic to advanced in a shorter format — perfect for beginners or working professionals looking for a strong foundation.
What is the Advanced Course?
The Advanced Course is a comprehensive, in-depth program designed to make you a subject matter expert. It includes extended hours, advanced content, and long-term professional support.
How do I choose between the Most Popular and the Advanced Course?
Choose the Most Popular Course if you want a quick, structured introduction.
Choose the Advanced Course if you’re aiming for deep expertise, career growth, or specialised roles.
Can I join both courses in one go?
Yes! You can enroll in both courses together to get the full learning experience and may be eligible for a combined discount.
Will this course help my job or exam?
Yes. Along with strong conceptual training, the course covers interview questions, practical case studies, and learning-outcome tests, helping you succeed in both professional roles and competitive exams.
Does it cover what I’m struggling with?
Yes, absolutely. We address individual doubts in detail and offer career guidance, so you can confidently move past what you’re struggling with.
What is the mode of training?
All training is conducted 100% online via live interactive sessions, with access to downloadable materials and expert support.
What is the duration of the Advanced Course?
The Advanced Course includes 12 sessions, totalling approximately 18 hours of expert-led training.
What is the duration of the Most Popular Course?
The Most Popular Course includes 5 sessions, totalling approximately 7.5 hours of training.
How do I choose among Every Pharma Professional, the Most Popular course and the Advanced Course?
Choosing the right PharmaGuru course depends on your career stage and goals. The Ever Pharm Professional Foundation Course is designed for career orientation, helping beginners build strong fundamentals and understand pharmaceutical industry pathways. The Most Popular Course focuses on skill development, equipping learners with practical, job-ready competencies. The Advanced Course is ideal for career acceleration, offering in-depth expertise and specialised training for professionals aiming to grow faster in their pharma careers.
Who provides the best pharma training courses in India?
PharmaGuru
Does PharmaGuru provide Online HPLC training for QC analysts?
Yes
Does PharmaGuru provide pharma training courses with placement guidance?
Yes
Need Help? Talk to a Course Advisor
- WhatsApp: +91-9910157319
- Email: admin@pharmaguru.co
Related Training:
- Corporate Pharmaceutical Training Programs | PharmaGuru
- Free Online Pharmaceutical Training | PharmaGuru
What Our Learners Say About PharmaGuru?
Trusted by students and professionals
⭐⭐⭐⭐⭐
Vivek Tiwari, Vadodara
“The Most Popular Course gave me exactly what I needed — In pharma knowledge forum very good learning of Analytical method development and Validation both. Good knowledge in very simple languag.”
⭐⭐⭐⭐⭐
Sunil Chaudhary, Nepal
“PharmaGuru’s Advanced Course was a game-changer. It’s a very fruitful platform for studying about the analytical method development by hplc GC and many more mainly I got knowledge from this about the method validation and is very useful platform for all users”
⭐⭐⭐⭐⭐
Sonali Mehendale – Munj, Delhi
“Thank you very much for conducting these modules for the course HPLC method development. It is being conducted very systematically and in a structured way. Your way of explanation along with case studies and industry application is very insightful.”
⭐⭐⭐⭐⭐
Dr Ankur Naik., Pharmaceutical Scientist, Vadodara
“What impressed me most was the 5-year professional support. Deep knowledge in simple words and effective presentation for better understanding. Thank you very much for sharing your valuable knowledge Sir.”
⭐⭐⭐⭐⭐
»Verified Google Review
“Excellent course structure, well-explained modules, and timely responses to queries. PharmaGuru truly understands what students need.”
