Polymer morphology and crystallinity are fundamental concepts that bridge molecular structure with the pharmacological performance of drug delivery systems. Polymer morphology studies the arrangement of polymer chains—whether amorphous (disordered), crystalline (ordered), or semi-crystalline (partly ordered). The degree of crystallinity determines key properties such as strength, stiffness, and density, and is influenced by factors like chain […]
Polymer morphology and crystallinity are fundamental concepts that bridge molecular structure with the pharmacological performance of drug delivery systems.
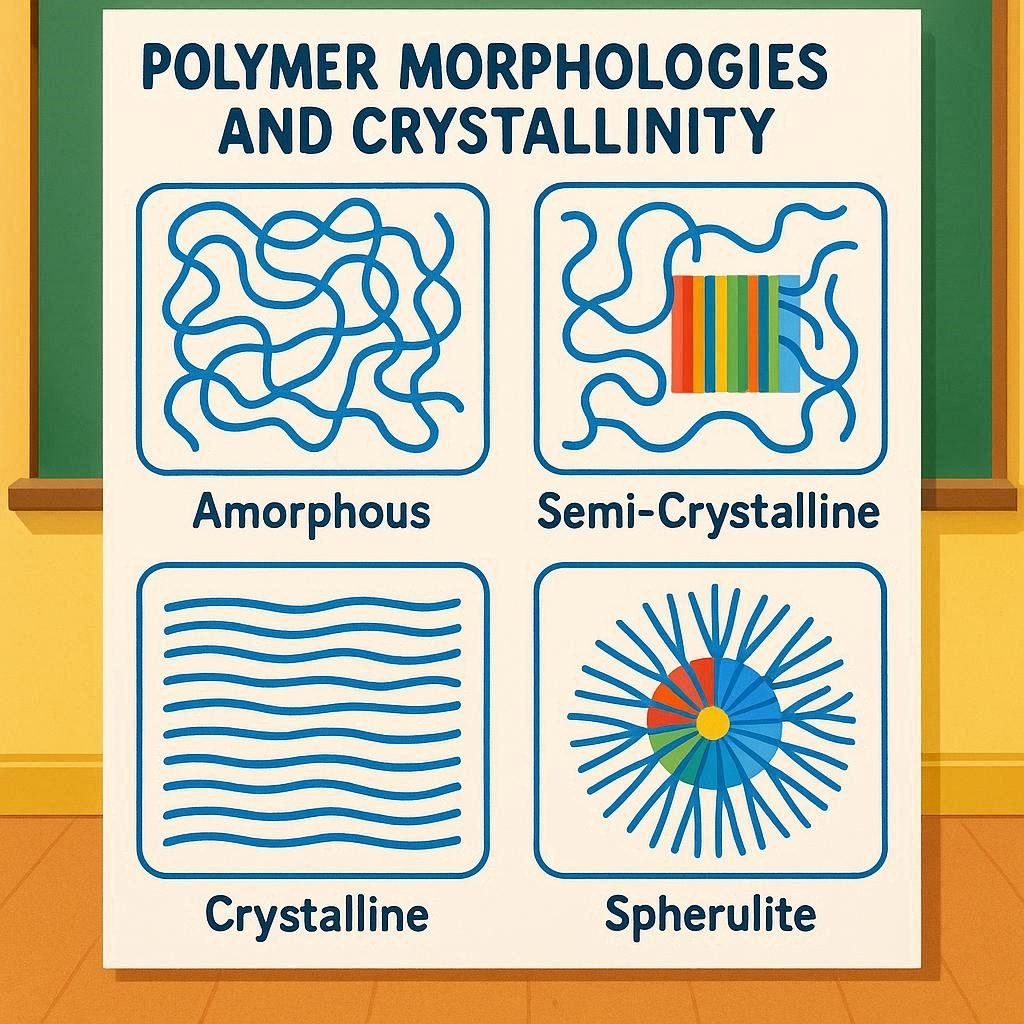
Polymer morphology studies the arrangement of polymer chains—whether amorphous (disordered), crystalline (ordered), or semi-crystalline (partly ordered). The degree of crystallinity determines key properties such as strength, stiffness, and density, and is influenced by factors like chain structure, molecular weight, and processing conditions (e.g., cooling rate).
| Topic | Description | Examples / Notes |
|---|---|---|
| 1. Introduction to Polymer Morphology | Polymer morphology refers to the internal structure and arrangement of polymer chains in the solid state. It determines many physical and mechanical properties. | Morphology bridges the gap between molecular structure and macroscopic behavior. |
| 2. Types of Polymer Morphology | There are two major morphological types: amorphous and crystalline. Real polymers often show a combination of both, known as semi-crystalline morphology. | Example: Polyethylene (semi-crystalline), Polystyrene (amorphous). |
| 3. Amorphous Polymers | Chains are randomly coiled and entangled without a long-range order. They behave like glassy or rubbery materials depending on temperature. | Features: Transparent, flexible, soft above glass transition temperature (Tg). |
| 4. Crystalline Polymers | Polymer chains are arranged in an ordered, repeating pattern forming crystalline regions or lamellae. | Features: Opaque, rigid, higher density and tensile strength. |
| 5. Semi-Crystalline Structure | Most polymers contain both crystalline and amorphous phases. The crystalline regions act as physical cross-links that enhance strength, while amorphous regions provide flexibility. | Example: Polypropylene, Nylon, PET. |
| 6. Factors Affecting Crystallinity | – Chain regularity (tacticity) – Cooling rate during processing – Molecular weight – Presence of plasticizers or additives | Isotactic polypropylene crystallizes easily; atactic polypropylene remains amorphous. |
| 7. Measuring Crystallinity | Common methods include X-ray diffraction (XRD), Differential Scanning Calorimetry (DSC), and Infrared Spectroscopy (IR). | DSC provides % crystallinity from melting enthalpy data. |
| 8. Morphological Structures | – Spherulites: Radial crystalline structures formed during cooling. – Lamellae: Thin plate-like crystalline regions. – Tie molecules: Connect crystalline and amorphous regions. | Spherulite size and density influence optical clarity and toughness. |
| 9. Effect on Properties | ||
| → Mechanical | Higher crystallinity → stronger and stiffer; lower crystallinity → softer and more ductile. | Example: HDPE (high crystallinity) vs LDPE (low crystallinity). |
| → Thermal | More crystalline polymers melt at higher temperatures due to stronger intermolecular forces. | Tm (melting temperature) increases with crystallinity. |
| → Optical | Amorphous → transparent; Crystalline → opaque due to light scattering. | PET bottles (semi-crystalline) are less transparent than amorphous PET films. |
| 10. Controlling Morphology in Processing | Processing conditions (cooling rate, stretching, annealing) can manipulate crystalline content and morphology. | Example: Slow cooling → higher crystallinity; rapid quenching → amorphous. |
| 11. Applications | Choosing the right morphology allows tailoring properties for specific uses. | – Amorphous: lenses, coatings, packaging – Crystalline: fibers, engineering plastics, containers |
| 12. Conclusion | Understanding and controlling polymer morphology and crystallinity is key to designing materials with desired mechanical, optical, and thermal properties. | Morphology = the hidden architecture of polymer performance. |
Polymer morphology refers to the internal structure, arrangement, and organisation of polymer chains in the solid state.
It describes how polymer molecules are packed and oriented, including whether they form ordered (crystalline) or disordered (amorphous) regions.
Example:
Polyethene exhibits semi-crystalline morphology, containing both crystalline lamellae and amorphous regions.
Crystallinity in polymers refers to the degree of structural order in which polymer chains are aligned in a regular, repeating pattern.
When segments of polymer chains pack together closely and uniformly, they form crystalline regions, while the remaining disordered parts form amorphous regions.
Effects on Properties:
| Property | Effect of Higher Crystallinity | Effect of Lower Crystallinity (More Amorphous) |
|---|---|---|
| Mechanical Strength | Increases (more rigid and tough) | Decreases (softer, more flexible) |
| Thermal Stability | Higher melting temperature (Tm) | Lower thermal resistance |
| Density | Higher, due to closer packing | Lower |
| Optical Clarity | More opaque (light scattering from crystalline regions) | Transparent |
| Permeability | Lower diffusion of gases/liquids | Higher permeability |
Example:
High-Density Polyethylene (HDPE) has greater crystallinity than Low-Density Polyethylene (LDPE), making it stronger and less flexible.
Several molecular and processing parameters affect how easily a polymer can crystallize:
| Factor | Influence on Crystallinity |
|---|---|
| Chain Regularity / Tacticity | Regular structures (isotactic, syndiotactic) promote crystallinity; irregular (atactic) chains hinder it. |
| Chemical Structure | Simple, symmetrical monomers crystallize easily (e.g., polyethylene); bulky or complex side groups disrupt packing. |
| Molecular Weight | Moderate molecular weights favor crystallization; very high weights lead to chain entanglement, reducing order. |
| Cooling Rate | Slow cooling allows time for chains to align → higher crystallinity; rapid quenching → amorphous structure. |
| Processing Conditions | Stretching, drawing, and annealing can enhance molecular orientation and crystallinity. |
| Additives / Plasticizers | Plasticizers increase chain mobility but often reduce crystallinity. |
| Feature | Amorphous Region | Crystalline Region |
|---|---|---|
| Structure | Random, disordered chain arrangement | Ordered, repeating pattern |
| Density | Lower | Higher |
| Optical Property | Transparent | Opaque |
| Thermal Transition | Shows a glass transition temperature (Tg) | Has a melting temperature (Tm) |
| Mechanical Behavior | Soft, rubbery above Tg | Hard, rigid |
| Molecular Mobility | High | Restricted |
Example:
In semi-crystalline polymers like polypropylene, crystalline lamellae are embedded within amorphous regions, forming a two-phase structure.
Crystallinity can be quantified using several analytical techniques:
| Method | Principle | Information Obtained |
|---|---|---|
| X-ray Diffraction (XRD) | Crystalline regions diffract X-rays at specific angles (Bragg peaks). | Determines degree and orientation of crystallinity. |
| Differential Scanning Calorimetry (DSC) | Measures heat absorbed during melting. The melting enthalpy (ΔHm) is proportional to % crystallinity. | % Crystallinity = (ΔHm / ΔHm⁰) × 100 |
| Infrared (IR) or Raman Spectroscopy | Monitors characteristic vibrational modes of ordered vs disordered chains. | Qualitative crystallinity assessment. |
| Density Measurement | Crystalline regions are denser; overall density correlates with crystallinity. | Simple but less precise. |
Crystalline polymers form unique hierarchical structures:
| Level | Structure Description |
|---|---|
| Lamellae | Thin, plate-like crystalline layers where chains fold back and forth. |
| Spherulites | Spherical aggregates of lamellae radiating from a nucleation point; visible under polarized light microscopy. |
| Tie Molecules | Chain segments connecting crystalline lamellae across amorphous regions, improving mechanical strength. |
Example:
When polyethene cools slowly from the melt, it forms spherulites composed of folded-chain lamellae separated by amorphous zones.
Crystallinity significantly influences thermal, mechanical, and barrier properties of polymers.
| Property Category | Influence of Crystallinity | Example |
|---|---|---|
| Mechanical | Higher crystallinity → stiffness, tensile strength, hardness; but lower elasticity. | HDPE vs LDPE |
| Thermal | Increases melting temperature and heat resistance. | Nylon-6 (high Tm) |
| Chemical Resistance | Improved solvent and moisture resistance. | Crystalline polymers resist swelling. |
| Optical | Light scattering by crystallites makes polymer opaque. | Crystalline PP = opaque, Amorphous PS = transparent. |
| Barrier | Reduced permeability to gases and water vapor. | Used in packaging films. |
| Parameter | Tg (Glass Transition Temperature) | Tm (Melting Temperature) |
|---|---|---|
| Definition | The temperature at which crystalline regions melt and the polymer becomes a viscous liquid. | Temperature at which crystalline regions melt and the polymer becomes a viscous liquid. |
| Applies To | Amorphous and semi-crystalline polymers (amorphous phase). | Only crystalline or semi-crystalline polymers (crystalline phase). |
| Reversibility | Reversible, second-order transition (no latent heat). | First-order transition (involves latent heat). |
| Molecular Mobility | Chains gain segmental motion. | Crystalline order completely breaks down. |
| Typical Values | Tg < Tm | Tm > Tg |
| Example | Polystyrene Tg ≈ 100 °C | Polyethylene Tm ≈ 130 °C |
Controlling polymer crystallinity is essential in pharmaceuticals because it directly impacts a drug’s release rate, mechanical stability, and bioavailability.
Highly crystalline polymers form dense, ordered structures that slow drug diffusion, making them ideal for controlled or sustained-release formulations.
Amorphous polymers, on the other hand, enhance drug solubility and dissolution rate, which is crucial for poorly water-soluble active ingredients.
By fine-tuning crystallinity through processing methods (like cooling rate or solvent evaporation), formulators can design drug delivery systems with predictable and reproducible performance.
Polymer morphology determines how a drug molecule is distributed and migrates through the polymer matrix.
1. In amorphous regions, molecular mobility and free volume are higher, allowing faster drug diffusion.
2. In crystalline regions, tightly packed chains create barriers to diffusion, leading to slower, sustained release.
Therefore, the ratio of amorphous to crystalline regions, along with their connectivity (via tie molecules), defines whether a formulation exhibits immediate, delayed, or sustained release.
Several processing and formulation techniques can be used to modify polymer morphology to achieve desired drug performance characteristics:
Solvent Casting or Spray Drying: Rapid solvent removal promotes amorphous structures, enhancing solubility.
Melt Extrusion or Annealing: Controlled heating and cooling allow adjustment of crystallinity for optimal mechanical strength.
Copolymerization: Introducing different monomers disrupts regular packing, reducing crystallinity and improving flexibility or drug compatibility.
Addition of Plasticisers or Additives: Alters molecular mobility and can reduce crystallinity for improved processing or drug dispersion.
By combining these methods, formulators can tailor drug release kinetics, physical stability, and bioavailability according to therapeutic requirements.
Polymer morphology and crystallinity are fundamental concepts that connect molecular structure to real-world performance.
By controlling crystallinity through polymer chemistry and processing, scientists can design materials with specific mechanical strength, flexibility, solubility, and thermal stability — critical in fields from packaging to pharmaceutical formulations.
Further Reading:
Quick Links