
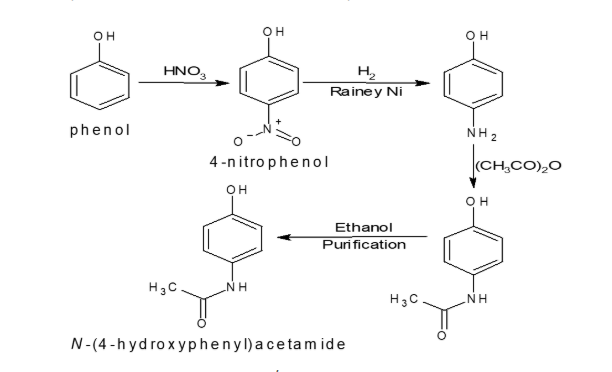
Route of Synthesis (ROS) In Pharmaceutical Development: Interview Questions What is the API route of synthesis? he API Route of Synthesis refers to the detailed sequence of chemical reactions and processes used to produce the Active Pharmaceutical Ingredient (API) from basic starting materials or intermediates. Key Points: What is a route scouting? Route scouting is […]
A Route of Synthesis (ROS) in pharmaceutical development is a carefully designed, step-by-step process for producing a drug from starting materials, based on key criteria such as efficiency, safety, cost-effectiveness, and scalability. It involves route scouting to identify the most optimal reaction pathway, often guided by retrosynthetic analysis, which works backward from the final product to simpler, readily available precursors. The ultimate goal is to synthesize the Active Pharmaceutical Ingredient (API) with high yield, purity, and consistency, ensuring the process is robust and suitable for large-scale manufacturing.
he API Route of Synthesis refers to the detailed sequence of chemical reactions and processes used to produce the Active Pharmaceutical Ingredient (API) from basic starting materials or intermediates.
Key Points:
Route scouting is the process of exploring and evaluating different possible synthetic pathways to produce a target compound, such as an Active Pharmaceutical Ingredient (API), during early drug development.
In short, route scouting helps select the most practical and economical synthetic method for drug development.
The principles/concepts of deciding a Route of Synthesis (ROS) in pharmaceutical development are based on scientific, economic, and regulatory considerations to ensure the process is efficient, safe, and scalable.
| Principle | Explanation |
|---|---|
| 1. Retrosynthetic Analysis | Break down the target molecule into simpler, commercially available precursors. |
| 2. Atom Economy | Minimise the number of reaction steps to reduce cost, time, and waste. |
| 3. Step Economy | Use inexpensive, readily available reagents and minimise costly purification. |
| 4. Yield and Purity | Choose routes that provide high yield and desired purity of the API. |
| 5. Safety and Environmental Impact | Avoid hazardous reagents and conditions; prefer green chemistry principles. |
| 6. Cost-Effectiveness | Use inexpensive, readily available reagents and minimize costly purification. |
| 7. Scalability | Ensure the route can be scaled from lab to industrial production. |
| 8. Stereoselectivity | Maintain or control stereochemistry, especially in chiral drugs. |
| 9. Regulatory Compliance | Ensure route avoids toxic or banned substances and meets quality standards. |
| 10. Impurity Control | Select routes that allow easy identification and control of impurities. |
These principles are often balanced together to find the most practical and robust synthetic route for a given pharmaceutical compound.
Related:
Quick Links