Human Errors In Pharmaceutical analysis: How to identify and minimize
Human error in pharmaceutical analysis refers to personnel mistakes that can compromise product quality, patient safety, and regulatory compliance. These errors usually arise from systemic factors—such as unclear procedures, poor communication, insufficient training, or equipment issues—rather than individual fault alone. Human Error Case Study: Real-Life Incident from a Pharmaceutical Laboratory Background Early in my career, […]
Route of Synthesis (ROS) In Pharmaceutical Development: Interview Questions
Route of Synthesis (ROS) In Pharmaceutical Development: Interview Questions What is the API route of synthesis? he API Route of Synthesis refers to the detailed sequence of chemical reactions and processes used to produce the Active Pharmaceutical Ingredient (API) from basic starting materials or intermediates. Key Points: What is a route scouting? Route scouting is […]
Quality Control (QC) Vs Quality Assurance (QA): Interview Questions

Quality Control (QC) is a product-focused and reactive approach that involves inspecting, testing, and measuring finished products to identify and correct defects after production. In contrast, Quality Assurance (QA) is a process-focused and proactive approach that aims to prevent defects by developing and implementing quality standards throughout the entire product lifecycle. In essence, QA builds […]
Pharmaceutical Dosage forms, Excipients and APIs In Drug Development: 35+ Inteview Questions
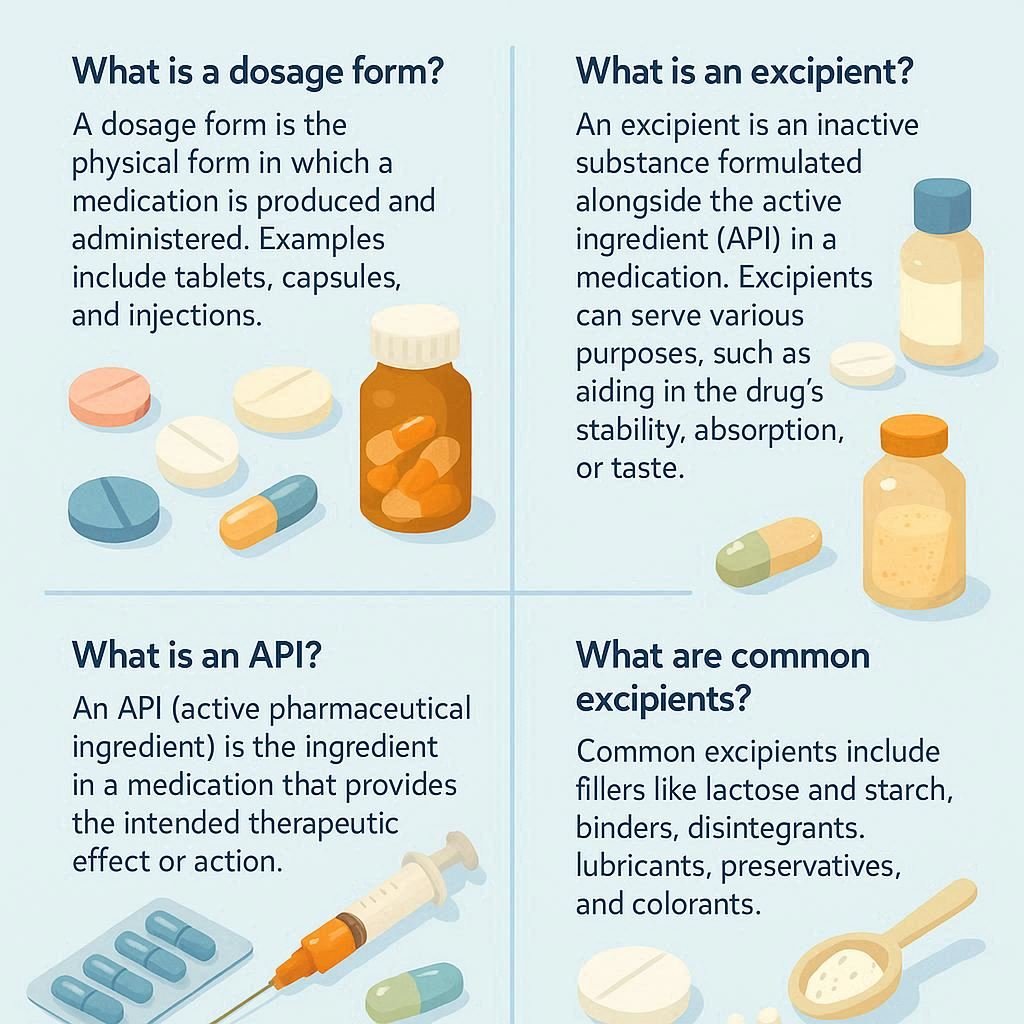
A pharmaceutical dosage form is the final physical presentation of a drug, containing both the active pharmaceutical ingredient (API) and excipients, with the API providing the therapeutic effect and excipients aiding in the formulation, stability, and delivery. Pharmaceutical Dosage forms, Excipients and APIs: Key Differences Aspect Pharmaceutical Dosage Forms Excipients Active Pharmaceutical Ingredients (APIs) Definition […]
ALCOA vs ALCOA+ vs ALCOA++: Get Mastery with 5+FAQs
The query “ALCOA vs ALCOA+ vs ALCOA++” refers to the evolving data integrity standards: ALCOA outlines the core principles—Attributable, Legible, Contemporaneous, Original, and Accurate; ALCOA+ adds Complete, Consistent, Enduring, and Available; and ALCOA++ further includes Traceable, emphasising a comprehensive audit trail. ALCOA vs ALCOA+ vs ALCOA++: FAQs ALCOA vs ALCOA+ vs ALCOA++ Principle ALCOA ALCOA+ […]
Pharmaceutical Intermediates vs APIs (Active Pharmaceutical Ingredients): 15+FAQs
Pharmaceutical intermediates are chemical building blocks used in the multi-step synthesis of APIs—the active drug components that provide therapeutic effects. Unlike APIs, intermediates are not administered to patients, have no therapeutic effect, and are subject to less stringent regulations. However, their quality directly impacts the safety and efficacy of the final API. Pharmaceutical Intermediates vs […]
Classification Of Analytical Methods For Pharmaceutical Analysis: Learn with 7+FAQs

Classification of Analytical Methods is based on classical (wet chemistry, e.g., titration, gravimetry) and instrumental (e.g., spectroscopy, electrochemistry), and by their goals: qualitative (identifying substances) and quantitative (measuring amounts). Separation techniques, like chromatography, form a key category used to isolate components before analysis. Major Takeaway You May Like Classification Of Analytical Methods For Pharmaceutical Analysis […]
MACO Calculation In Pharmaceuticals: Learn With 3+ Case Studies and 7+FAQs
The MACO calculation, or the Maximum Allowable Carryover calculation in pharmaceutical manufacturing, determines the maximum residue from a previous product that can remain on shared equipment without risking contamination or harm to the next product or patient. It is essential for cleaning validation to ensure effective cleaning and prevent cross-contamination. FAQs on MACO Calculation […]
C18 and C8 HPLC Columns: Why Are They Crucial For Pharmaceutical Analysis

Both C18 and C8 HPLC columns are the heart of the pharmaceutical analysis due to their longer life, wider applications, better selectivity and high theoretical plate. The C8 column (Octyl column) is a reversed-phase chromatographic column with an 8-carbon alkyl chain bonded to a silica support, used in HPLC for separating compounds based on hydrophobicity. […]
Related Substances vs Assay Test in Pharmaceuticals: Interview Questions

Related substances and assay tests are critical quality control measures in pharmaceuticals. The related substances test detects and quantifies impurities, while the assay test measures the active pharmaceutical ingredient (API) content. Together, they ensure the drug’s purity, potency, safety, and overall quality. Related Substances vs Assay Test in Pharmaceuticals: Key Differences Parameter Related Substances Test […]
