earn Gas Chromatography (GC) calibration including parameters, step-by-step procedures, frequency, acceptance criteria, detector precision, linearity, and FAQs for accurate and compliant GC analysis.
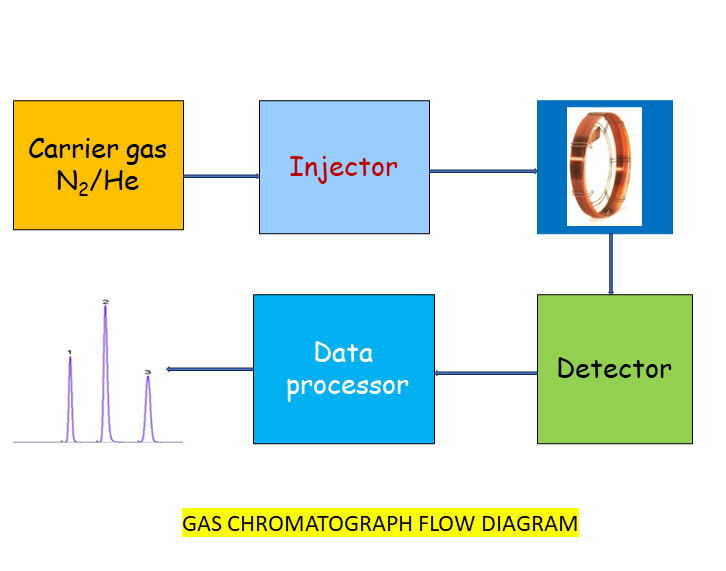
Gas Chromatography (GC) calibration ensures accurate and reliable chemical analysis by verifying critical instrument components such as column oven temperature, gas flow rates, and detector performance. Calibration also involves generating a calibration curve by plotting detector response against known concentrations to quantify unknown samples.
GC calibration is typically performed using certified reference standards, evaluating parameters like linearity, precision, and detector response, and confirming system suitability. This process is essential in regulated industries such as pharmaceuticals, chemicals, food testing, and environmental analysis, where data integrity and compliance are critical.
Related: Pharmaceutical Analysis
The following 9 parameters are evaluated during Gas Chromatograph calibration:
Gas Chromatograph calibration should be performed:
The following reagents and instruments are required and must have a valid Certificate of Analysis (CoA):
Acceptance Criteria:
Verify the flow rates of gases using a calibrated flow meter:
| Gas | Acceptance Limit |
|---|---|
| Carrier Gas (N₂ / He) | 22.5 – 27.5 ml/min |
| Hydrogen | 36 – 44 ml/min |
| Air | 360 – 440 ml/min |
| Parameter | Setting |
|---|---|
| Oven Temperature Program | 40°C (0 min) → 25°C/min → 90°C → 15°C/min → 170°C (15 min) |
| Injector Temperature | 250°C |
| Detector (FID) Temperature | 270°C |
| Carrier Gas Flow | 0.5 ml/min |
| Split Ratio | 10:1 |
| Hydrogen Flow | 40 ± 4 ml/min |
| Zero Air Flow | 400 ± 40 ml/min |
| Auxiliary Gas (N₂) | 25 ± 2.5 ml/min |
| Injection Volume | 1.0 µl |
| Septum Purge Flow | 5 ml/min |
| Syringe Capacity | 10 µl |
Prepare five standard solutions in 10 ml volumetric flasks:
| Concentration | Stock Solution Volume |
|---|---|
| 100 µg/ml | 1.0 ml |
| 200 µg/ml | 2.0 ml |
| 300 µg/ml | 3.0 ml |
| 400 µg/ml | 4.0 ml |
| 500 µg/ml | 5.0 ml |
Make up to volume with n-Hexane.
| Compound | Relative Retention Time (RRT) |
|---|---|
| n-Hexane | ~0.41 |
| n-Tetradecane | 1.00 |
| n-Pentadecane | ~1.16 |
| n-Hexadecane | ~1.39 |
Retention time of n-Tetradecane: ~10.5 minutes
Acceptance Criteria:
Related Topics:
Gas Chromatography calibration is a critical quality assurance activity that confirms instrument accuracy, precision, and linearity. Proper calibration ensures reliable quantitative results, regulatory compliance, and confidence in analytical data, especially in GMP and regulated laboratory environments.
The following steps are performed in GC calibration:
The parameters like Column-oven temperature, Flow rate of the gases, Detector performance, Detector precision and Detector linearity are performed in Gc calibration.
In detector precision, the Percentage RSD of the area response of the peaks corresponding to n-Pentadecane and n-Hexadecane to that of n-Tetradecane in the chromatograms of six replicatem injection should not be more than 2.0%.
Once in a six months ± 5 days or any failure
The correlation coefficient (r2) should be more than 0.99
n-Hexane, n-Tetradecane, n-Pentadecane and n-Hexadecane are used in GC calibration
Calibration of a Gas Chromatograph (GC) is essential to ensure the accuracy, precision, and reliability of analytical results. GC calibration verifies that critical system parameters—such as column oven temperature, carrier gas flow rates, injector and detector temperatures, and detector response—are functioning within specified limits.
Without proper calibration, variations in temperature or gas flow can lead to shifts in retention time, poor peak resolution, inaccurate quantification, and unreliable detector response. Calibration also confirms detector linearity and precision, ensuring that the GC produces a proportional and reproducible response across a defined concentration range.
In regulated environments such as pharmaceutical, food, environmental, and petrochemical laboratories, GC calibration is a mandatory requirement under GMP, GLP, and ISO standards. Proper calibration supports data integrity, audit readiness, method validity, and compliance with regulatory expectations, ultimately ensuring confidence in reported analytical results.
Great — below are the remaining 10 detailed FAQs, written in a clear, SEO-friendly, and regulatory-compliant style. Together with the earlier one, these complete the 11 FAQs section.
A Gas Chromatograph should be calibrated once every six months (±5 days) as part of routine preventive maintenance. Calibration must also be performed after major repairs, replacement of critical components (column, detector, flow controller), or when system performance fails acceptance criteria.
GC calibration is commonly performed using certified reference standards such as n-Hexane, n-Tetradecane, n-Pentadecane, and n-Hexadecane, depending on the detector and application. All standards must have a valid Certificate of Analysis (CoA) and be prepared using suitable analytical-grade solvents.
Detector precision evaluates the repeatability of the detector response. It is assessed by injecting a standard solution multiple times (typically six injections) and calculating the %RSD of peak area ratios. Low %RSD values indicate consistent detector performance and stable system conditions.
Detector linearity measures the ability of the detector to produce a response directly proportional to analyte concentration across a defined range. It is established by injecting multiple concentration levels and plotting a calibration curve. A correlation coefficient (r² ≥ 0.99) confirms that the GC can accurately quantify unknown samples.
If calibration acceptance criteria are not met, the GC must be considered out of calibration. Analytical testing should be stopped, the issue investigated, corrective actions implemented (e.g., maintenance, recalibration), and calibration repeated. Data generated during the failure period may require impact assessment or invalidation.
Yes. GC calibration is mandatory in pharmaceutical laboratories under GMP, GLP, USP, EP, and ISO guidelines. Regulatory agencies require documented evidence that analytical instruments are calibrated, qualified, and fit for intended use to ensure data accuracy and patient safety.
GC calibration verifies the instrument’s accuracy and performance against known standards, while system suitability testing ensures that the entire analytical system (instrument, method, column, reagents) is performing adequately before sample analysis. Calibration is periodic; system suitability is typically performed before each analytical sequence.
GC calibration data support method validation but do not replace it. Calibration confirms instrument performance, while method validation evaluates parameters such as accuracy, precision, specificity, linearity, robustness, and range of the analytical method under defined conditions.
GC calibration records should include raw data, calculations, chromatograms, acceptance criteria, results, deviations, corrective actions, analyst signature, and review approval. Proper documentation ensures traceability, audit readiness, and compliance with data integrity principles (ALCOA+).
Further reading:
Quick Links